Interview Summary
Endometrial cancer is the most common gynaecological malignancy in developed countries, and often presents at an early stage. Paclitaxel plus carboplatin is the standard first-line chemotherapy for endometrial cancer; however, there is new evidence that the combination of chemotherapy and immunotherapy has synergistic effects in the treatment of this disease. For this article, EMJ conducted an interview in August 2023 with two key opinion leaders: Jubilee Brown and Wendel Naumann from Atrium Health Levine Cancer Institute, Charlotte, North Carolina, USA, both of whom have a wealth of experience and expertise in the management of endometrial cancer. The experts gave valuable insights into recent developments in endometrial cancer research as presented at the Society of Gynecologic Oncology (SGO) Annual Meeting on Women’s Cancers 2023, held on 25th–28th March 2023 in Tampa, Florida, USA, and online. Topics discussed included the unprecedented progression-free survival (PFS) data from two Phase III randomised controlled trials evaluating first-line immunotherapy in combination with chemotherapy in patients with advanced or recurrent endometrial cancer, RUBY with dostarlimab, and NRG GY018 with pembrolizumab, which created a buzz at SGO 2023. Clinically meaningful benefit of dostarlimab or pembrolizumab in combination with chemotherapy versus standard-of-care chemotherapy was seen regardless of mismatch repair status in RUBY and NRG-GY018, respectively. Brown and Naumann considered the implications of these results on first-line treatment and recurrent settings, and outlined the management of immune-related adverse events related to immunotherapy-based treatment regimens. The experts also explored key earlier stage studies presented at SGO, and the potential for personalised medicine in endometrial cancer. Finally, Brown and Naumann described what the future of the management of patients with endometrial cancer might look like, which clinical trials are needed, and which advancements in research they would like to see.INTRODUCTION
Endometrial cancer is the most common gynaecological cancer in high-income countries, and its incidence is increasing worldwide.1 This cancer often presents at an early stage, when the disease is still confined to the uterus.2 For a long time, endometrial cancer care has been plagued with poor outcomes for patients with advanced stage disease.3,4 Identifying endometrial cancer at an early stage is associated with better outcome,1 but there is currently no public health screening programme for this type of cancer.5,6 The lack of routine screening for endometrial cancer is particularly impactful in cases of high-grade, difficult-to-treat cancers, such as serous carcinoma and malignant mixed Müllerian tumour (MMMT; also known as carcinosarcoma), which often recur, even in early stage disease.7,8 Patients with Lynch syndrome are identified as being at risk for endometrial cancer, but these patients represent only a few of those at risk for this disease.9 Only females symptomatic for endometrial carcinoma (e.g., presenting with abnormal bleeding) typically seek medical help for its diagnosis and treatment.10
Establishing care with a gynaecological oncologist for management of gynaecological cancers is associated with improved outcomes;11 however, access to care for patients with endometrial cancer is a substantial issue globally. Barriers to care include lack of knowledge on endometrial cancer; poor communication; and clinical, administrative, financial, geographical, and facility-related difficulties.12 Further concerns surrounding endometrial cancer include disparities in clinical trial involvement13-15 and patient outcome.16 Furthermore, there are higher rates of more aggressive cancer in Black patients compared with White patients.17 To understand and address these disparities requires further research in which diverse populations are represented.
Standard first-line chemotherapy for endometrial cancer is paclitaxel plus carboplatin;18 however, the combination of chemotherapy and immunotherapy has recently gained considerable research interest in the gynaecological oncology community.18,19
EVOLVING TO A MOLECULAR-BASED STRATIFICATION IN ENDOMETRIAL CANCER
Naumann explained that the staging of endometrial cancer is evolving from anatomic staging and risk stratification to a more molecular-based stratification, which happened for breast cancer. In the past, patients with endometrial cancer were grouped together, and much of the data on this disease were derived from a variety of tumour types; however, serous and clear cell carcinomas are distinctly different to endometrioid cancers, and should be considered separately. This requirement indicates the need for well-planned molecular studies for molecular staging, assigning treatment, and defining prognosis, in line with the International Federation of Gynaecology and Obstetrics (FIGO) staging in endometrial cancer.20 Naumann specified that unmet needs for endometrial cancer include identifying risk factors for patients at high risk for disease recurrence, and de-escalating therapy for patients at lower risk, such as those with polymerase epsilon exonuclease mutated endometrial cancer.21
UNPRECEDENTED PROGRESSION-FREE SURVIVAL RESULTS FROM RUBY WITH DOSTARLIMAB AND NRG-GY018 WITH PEMBROLIZUMAB
According to Brown: “What was so striking to everyone in the audience at SGO 2023, including patients, was the game-changing outcomes in RUBY19 and NRG‑GY018.”18 Historically, chemotherapy alone has had limited responses in patients with endometrial cancer. The addition of immunotherapy to chemotherapy, followed by immunotherapy maintenance, led to a marked improvement in PFS, the primary endpoint in both of these studies.18,19 Notably, patients with difficult-to-treat MMMTs were included in RUBY.19 Furthermore, the improvement in PFS with immunotherapy appears to be sustained over time, as the Kaplan–Meier curves never converge (Figures 1 and 2).18,19 Importantly, the combination chemotherapy and immunotherapy regimens in RUBY19 and NRG-GY01818 were well tolerated, with no new safety signals. Naumann observed that in both trials there was a plateau in disease recurrences after the first year of treatment in the mismatch repair deficient (dMMR) subgroups, and considered that these were two of the most striking trials ever seen in gynaecological oncology research.
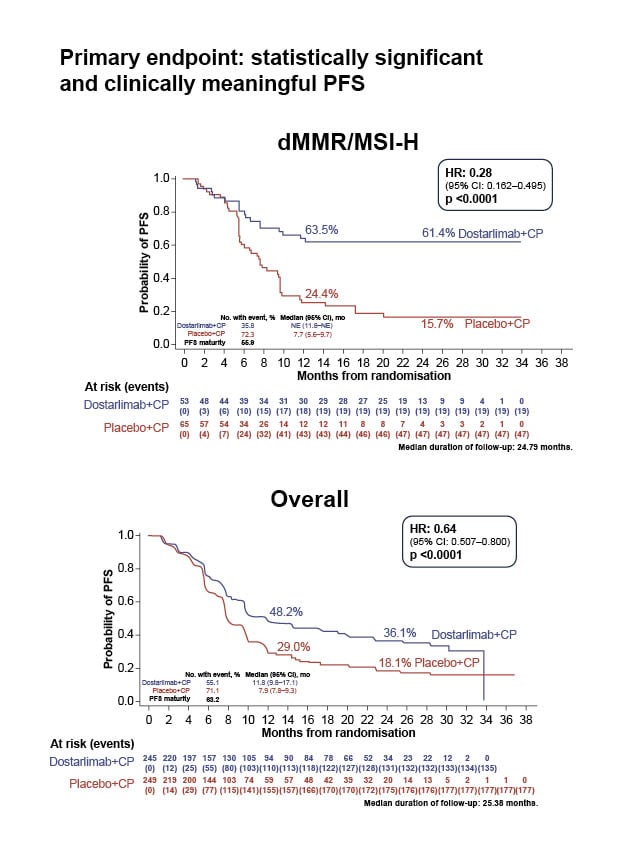
Figure 1: Statistically significant and clinically meaningful progression-free survival in RUBY.
Mirza et al.19
CI: confidence interval; dMMR: mismatch repair deficient; CP: carboplatin–paclitaxel; HR: hazard ratio; mo: month; MSI-H: microsatellite instability-high; NE: not evaluable; No.: number; PFS: progression-free survival.
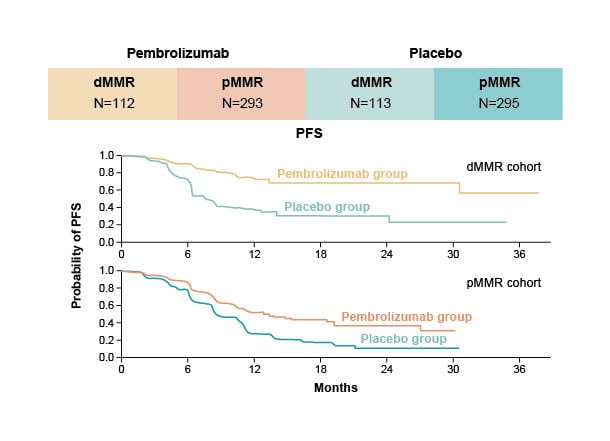
Figure 2: Significantly longer progression-free survival than with chemotherapy alone in NRG-GY018.
Eskander et al.18
dMMR: mismatch repair deficient; PFS: progression-free survival; pMMR: mismatch repair proficient.
CLINICALLY MEANINGFUL PROGRESSION-FREE SURVIVAL BENEFIT REGARDLESS OF MISMATCH REPAIR STATUS
Clinically meaningful improvements in PFS were seen in both the population with dMMR tumours and the population with mismatch repair proficient (pMMR) tumours, with the results being more impressive in the population with dMMR/microsatellite instability-high (MSI-H) in both studies.18,19 Brown described the hazard ratios (HR) for PFS in these trials as remarkable, with 0.28 (95% confidence interval [CI]: 0.16–0.50; p<0.001) in RUBY19 and 0.30 (95% CI: 0.19–0.48; p<0.001) in NRG‑GY01818 in the populations with dMMR/MSI-H.
Naumann explained that the results from RUBY19 and NRG-GY01818 cannot be compared directly because the studies included very different patient populations. RUBY19 was the more inclusive trial, for which considerably more high-risk patients, such as those with MMMT, clear cell carcinoma, serous carcinoma, and recurrence within 6 months of adjuvant chemotherapy, were eligible. In contrast, NRG-GY01818 excluded patients with these high-risk tumour types and patients with adjuvant chemotherapy within the previous 12 months.
According to Naumann, many in the research community were surprised to see such a dramatic improvement in PFS in the population with pMMR, shown by a 46% reduction in disease progression in NRG-GY01818 and a 24% decrease in RUBY.19 Brown described that in the pMMR cohort in NRG-GY018,18 median PFS was 13.1 months with pembrolizumab versus 8.7 months with placebo (HR: 0.54; 95% CI: 0.41–0.71; p<0.001), which, although less impressive than the results for the dMMR cohort (HR: 0.30), was still a statistically and clinically significant difference, and this difference was not expected.
Naumann stated that the most striking, and unexpected, aspect of the results from these studies was that the Kaplan–Meier curves flattened out for both the population with dMMR tumours and the population with pMMR tumours (Figures 1 and 2). Brown concurred and illustrated how physicians are used to seeing patients respond for a short time, then recur, or die, and this is not what was happening in these trials: the improvement was sustained, which showed the impact of immunotherapy, and explains the impressive HRs.
PROGRESSION-FREE SURVIVAL BENEFIT IN RUBY AND NRG-GY018 MAY TRANSLATE INTO AN OVERALL SURVIVAL BENEFIT
Overall survival (OS) was a primary endpoint in RUBY,19 and a secondary endpoint in NRG-GY018.18 The OS data from these studies are not yet mature. However, there are signs from RUBY19 that the observed PFS benefit may translate into an OS benefit, with a 24-month HR of 0.64 (95% CI: 0.46–0.87), which Brown described as really impressive. Naumann clarified that, from a purist standpoint, this result was not statistically significant because of the α-spin assigned to this endpoint (p=0.0021; α-spin=0.0017), but considered there was “no doubt that, in the end, we will see an OS benefit in these trials.”
IMPACT OF THE RESULTS OF RUBY AND NRG-GY018 ON FIRST-LINE TREATMENT OF ENDOMETRIAL CANCER
The impressive results from RUBY19 and NRG-GY01818 presented at SGO led to a change in the standard-of-care first-line treatment for endometrial cancer.22,23 Dostarlimab plus chemotherapy for patients with dMMR tumours in the first-line received U.S. Food and Drug Administration (FDA) approval on 31st July 2023,23 and approval in Europe based on these data is expected to follow, with use determined by local guidelines.
Brown remarked that although many physicians changed treatment practices immediately after the results of RUBY19 and NRG-GY01818 were announced at SGO 2023, controversially, a few institutions did not adopt the concept of adding immunotherapy to chemotherapy before adjuvant chemotherapy, because the OS benefit in RUBY19 was not technically statistically significant. However, most of the community interpreted these data as showing a marked improvement in the initial setting. Naumann noted that, historically, survival in endometrial cancer was approximately 12 months,24 whereas landmark OS at 24 months was 71.3% (95% CI: 64.5–77.1) with dostarlimab in RUBY.19 Furthermore, Brown noted, in the population with dMMR/MSI-H in RUBY, estimated PFS at 24 months was 61.4% (95% CI: 46.3–73.4) in the dostarlimab group, indicating that the median had not been reached.19
Naumann stated: “I cannot imagine that physicians will not adopt this new treatment approach for eligible patients as the data are so compelling, and there are two randomised trials that confirm the findings. The PFS and OS benefits observed with this treatment combination are hard to ignore.” Naumann also commented that the data are so remarkable that there is now debate about whether immunotherapy should be administered with adjuvant chemotherapy in high-risk patients, particularly in the population with dMMR, even before the release of results from KEYNOTE-B21, which comprises pembrolizumab in combination with adjuvant chemotherapy with or without radiotherapy in participants with newly diagnosed endometrial cancer after surgery with curative intent.25
The positive results seen in RUBY19 and NRG-GY01818 are “hoped for in every trial but are rarely achieved,” according to Brown, who also described how moving it was to be sitting in the audience at SGO when the results were presented: “There were standing ovations, tears, and a sense of gratitude for all the patients who participated in the trials, and for the physicians who dedicate their time and effort to the running of the trials. These results and the changes to standard of care are going to impact so many people, and likely save many lives.”
IMPLICATIONS OF THE RESULTS OF RUBY AND NRG-GY018 ON SECOND-LINE THERAPY IN PATIENTS WITH ENDOMETRIAL CANCER
How the results of RUBY19 and NRG-GY01818 will impact second-line care in patients with endometrial cancer is “the million dollar question,” according to Naumann, because frontline data have changed practice. For patients with pMMR, combination of the tyrosine kinase inhibitor, lenvatinib, with pembrolizumab is a standard option and an approved second-line therapy.26 Naumann acknowledged that it is more difficult to decide which second-line therapy to provide for patients with dMMR; however, the hope is that the majority of these patients will not have disease recurrence. If there is recurrence, treatment options may include administering the same immunotherapy as used in first-line (with or without chemotherapy), or utilising other immunotherapy regimens, such as lenvatinib-pembrolizumab, or immunotherapy plus a vascular endothelial growth factor (VEGF) inhibitor, but there are no clinical study data to guide these treatment decisions. There is a clear need to research, define, and optimise second-line regimens for this patient population.
Brown emphasised that there are no data on the use of immunotherapy in the recurrent setting after first-line immunotherapy in endometrial cancer; therefore, it is unknown whether it is safe and effective to re-use immunotherapy combinations, and in which order they should be administered. The experts clarified that they would never withhold immunotherapy from a patient just because they might need it in the recurrent setting, because the outcome is so positive with the immunotherapy-chemotherapy combination in first-line.
MANAGEMENT OF IMMUNE-RELATED ADVERSE EVENTS RELATED TO IMMUNOTHERAPY-BASED TREATMENT REGIMENS
Brown highlighted that immunotherapy is being increasingly used in oncology, and over the last 3–5 years, gynaecological and medical oncologists have become much more familiar with this therapy, and the management of immune-related adverse events. For example, healthcare professionals are familiar with managing colitis by interrupting treatment, or initiating steroids or other medications. Furthermore, there are clear American Society of Clinical Oncology (ASCO)27 and National Comprehensive Cancer Network (NCCN)28 guidelines on management of immune-related adverse events related to immunotherapy-based treatment.
Naumann underscored that most patients do not have significant toxicities associated with immunotherapy, reiterating that there were no new safety signals in RUBY19 and NRG-GY018.18 These are standard drugs that are well tolerated by most patients, with treatment discontinued for immunotherapy-related toxicity in only approximately 8% of patients.19 Therefore, patients are usually able to continue their therapy, and adverse events that occur are generally manageable. Naumann emphasised that it is essential for consultants to be familiar with treatment-related toxicities, and to have the knowledge to educate and counsel their patients effectively to ensure they monitor their symptoms (e.g., daily for colitis) and help them manage any severe toxicities. Some patients might receive immunotherapy-based treatments administered in community healthcare settings that have no consultants with experience in managing these toxicities. In this case, Naumann considered it vital that the community establishments develop relationships with institutions that have this type of expertise, to advise them on how to manage immune-related adverse events.
OTHER KEY CLINICAL STUDIES IN ENDOMETRIAL CANCER PRESENTED AT THE SOCIETY OF GYNECOLOGIC ONCOLOGY
Naumann highlighted NRG25829 as a study of particular interest at SGO. This was a randomised trial of chemotherapy and radiation compared to radiation alone in patients with Stage III/IVa endometrial cancer with <2 cm of residual tumour, or Stage I/II clear cell or serous cancer with positive cytology.29 The stratified HR for death for chemotherapy plus radiation versus radiation alone was 1.05 (95% CI: 0.82–1.34; p=0.72).29 There were no subgroups (age, BMI, stage, residual disease) that predicted an OS benefit from the combined chemotherapy and radiation treatment.29
KEY RESEARCH IN MOLECULAR PROFILING AND BIOMARKER TESTING FOR ENDOMETRIAL CANCER PRESENTED AT THE SOCIETY OF GYNECOLOGIC ONCOLOGY
Outcome data from the Endometrial Cancer Molecularly Targeted Therapy Consortium (EMTC2) were reported based on molecular profiling of the endometrial tumours.30 Patients with tumours that were both tumour mutational burden high (TMB-H) and dMMR had excellent outcomes, and median OS was not reached in this cohort. Patients with TMB-H tumours had a median survival of 113 months compared with 88 months for patients with dMMR tumours, and 43 months for patients whose tumour was neither dMMR nor TMB-H. Notably, 91% (n=149) of Black patients were neither TMB-H nor dMMR compared with 76% (n=481) of non-Black patients (p=0.001). In addition, Black patients had a significantly lower rate of dMMR than non-Black patients (6% versus 18%; p=0.001).30 Naumann stated that these factors may explain the worse prognosis in Black patients.
CCNE1 gene amplification and human epidermal growth factor receptor 2/neu (HER2) positivity have also been associated with poor prognosis in endometrial cancer.31 A single institution study showed significant racial differences in these biomarkers.31 Black patients were significantly more likely than non-Black patients to have CCNE1 gene amplification (26% versus 4%; p<0.001) and HER2 positivity (22% versus 15%; p=0.106).31 Both markers were present in 9% of Black patients compared with only 1% of non-Black patients (p<0.001).31 This dual molecular signature was associated with a significantly worse OS than no dual molecular signature (43 months versus 161 months; p=0.05).31
COMPARING IMMUNOTHERAPY VERSUS CHEMOTHERAPY IN THE FIRST-LINE SETTING IN PATIENTS WITH ENDOMETRIAL CANCER
Studies to compare immunotherapy versus chemotherapy in the first-line setting in patients with advanced or recurrent endometrial cancer, such as KEYNOTE-C93,32 DOMENICA,33 and LEAP-00134 are recruiting, or ongoing. Naumann identified that these studies do not have the new standard of care (i.e., chemotherapy plus immunotherapy) as a control arm, so they may be difficult to interpret, but they are still very important. These studies will provide data on immunotherapy alone versus chemotherapy alone, thereby helping to guide next steps in research, and potentially increasing treatment choice, perhaps including non-chemotherapy-based treatment, for patients. Naumann and Brown noted that future studies may comprise chemotherapy plus immunotherapy versus immunotherapy alone, but are unlikely to include chemotherapy alone, as adding immunotherapy to chemotherapy makes such a huge difference to response.
PERSONALISING CARE FOR PATIENTS WITH ENDOMETRIAL CANCER
Naumann expressed that the results from RUBY19 and NRG-GY01818 apply to all patients with endometrial cancer, which is really good news. In terms of personalising care, Naumann considered that, following the success of immunotherapy-based treatment in patients with dMMR, future research should address how to improve outcomes in patients with pMMR; for example, adding a VEGF inhibitor or a tyrosine kinase inhibitor to boost response in a way that does not induce excess toxicity. In addition, data from KEYNOTE-B2125 will be important for defining the optimal adjuvant regimen for patients at high risk, including those with Stage IIIc non-measurable disease, who were excluded from RUBY19 and NRG-GY018.18
Brown is looking forward to the subset analyses from trials such as RUBY19 and NRG-GY01818 to inform personalised medicine for patients with rare histological subtypes, such as clear cell or MMMT, or specific molecular profiles. In patients with advanced or recurrent uterine serous carcinomas that overexpress HER2, the addition of trastuzumab to carboplatin-paclitaxel yielded excellent outcomes (median PFS, all patients: 8.0 months for carboplatin-paclitaxel versus 12.6 months for carboplatin-paclitaxel-trastuzumab; p=0.005; HR: 0.44; 90% CI: 0.26–0.76).35 Brown raised the question whether these patients should be treated with trastuzumab or immunotherapy in combination with chemotherapy, and indicated that further research is required on this topic.
FUTURE PROSPECTS AND CONCLUSIONS
Brown would like to see further research into the use of immunotherapy, in the light of the huge PFS benefit shown in RUBY19 and NRG-GY018,18 and looks forward to the results of ongoing trials in the first-line setting. Combination of immunotherapy with other agents, and efforts towards personalised treatment for patients with rare histological subtypes and specific molecular profiles, are also important areas for future research. Brown emphasised that it is essential that all patients with endometrial cancer have access to these therapies.
Naumann pointed out that there are many immunotherapy options that have not yet been investigated in patients with endometrial cancer, and questioned whether outcomes in patients with dMMR can be improved. Although the addition of lenvatinib might not be particularly beneficial in this patient population, there are other molecules that might be beneficial. For example, the role of VEGF inhibitors in endometrial cancer needs to be determined. Naumann highlighted the need to define and optimise second-line care for patients with dMMR, and to optimise treatment in patients with pMMR, to enable treatment responses similar to those seen in patients with dMMR.







