Abstract
Myopericytoma (previously defined haemangiopericytoma) is a rare vascular tumour originating from extracapillary cells called pericytes and rarely occurs in paranasal sinuses. Surgical wide resection is the treatment of choice. Post-operative radiotherapy is usually used in the case of incomplete resection. The current study reports a case of a 38-year-old male with an extended maxillary sinus myopericytoma, who developed an early bulky recurrence 3 months after surgical excision. The relapse was treated using salvage radiation therapy with radical purpose. Radiotherapy was completed 52 months ago, at the time of writing, and the patient is alive and disease-free. Although literature data report radioresistance as an important obstacle for management of this tumour, it could be important to start considering myopericytoma as a heterogeneous entity, with different underlying molecular mechanisms, growth kinetics, and response to treatments. High-dose ‘modern radiotherapy’ with radical purpose represents a reliable treatment strategy in cases with no surgical option. Furthermore, given the paucity of data available in the literature regarding this clinical setting, the authors conducted an overview on the topic.
Key Points
1. Sinonasal myopericytoma is an uncommon neoplasm derived from pericytes surrounding the blood vessels and, due to its rarity, there are no standardised treatments.2. Clinical data regarding the use of definitive radiotherapy as primary treatment modality in sinonasal myopericytoma are scarce.
3. High-dose definitive radiotherapy using a modern technique should be considered for sinonasal myopericytoma cases where the tumours are unresectable or the patient is unfit for surgery.
INTRODUCTION
Myopericytoma (MPC) is a term used to describe a continuum of mesenchymal tumours with elevated cellularity found in bone and soft tissue. Previously called haemangiopericytoma, MPC is an uncommon neoplasm derived from pericytes surrounding the blood vessels and rarely arises in the nasal cavity and paranasal sinuses, accounting <0.5% of all neoplasms.1 MPC has been reported at all ages, but there appears to be an incidence peak in the fifth and sixth decades of life. Spontaneous or induced bleeding and nasal obstruction are the main complaints in MPC of the nose and paranasal sinuses. Pain can occur when lesions invade surrounding tissues or are confined in unyielding spaces such as the paranasal sinuses. MPC typically presents as a slow-growing, solitary mass exhibiting low malignant potential. Although many reports describe this tumour as indolent, its potential for local invasion and destruction of adjacent structures as well as for metastatic spread has been reported.2 Aggressive behaviour predictors include tumour size of >5 cm, bone invasion, profound nuclear pleomorphism, increased mitotic activity (>4/10 high power fields), presence of immature tumour cells, necrosis, foci of haemorrhage, and a proliferation index of >10%.1 However, relapse and metastases can occur even in cases of more favourable histological appearances.3
Scientific literature on sinonasal MPC is scant, and it consists primarily of small case series and case reports. Due to its rarity, there are no standardised treatments. Radical surgery (performing en bloc resection with wide margins) is considered the standard of care.2,4 Pre-operative embolisation is often employed to reduce intra-operative blood loss. Adjuvant treatment remains largely empiric. Because there is a risk of relapse of up to 30% after surgery, some chemotherapy regimens, including anthracycline and ifosfamide agents, as well as post-operative radiotherapy (RT) have been proposed.5 Traditionally, MPC is considered intrinsically resistant to systemic therapy and with low radiosensitivity. However, several adverse features such as high mitotic activity, high proliferation index, and rich vascularisation could potentially overcome its radioresistence.
In this context, the role of definitive RT as primary modality remains unclear. Definitive RT has been explored in a small number of sinonasal MPC cases, but outdated RT techniques and improper low total doses were used.4 Probably, ‘modern RT’ can play a role in this oncological scenario, assuring higher lethal dose to the target volume, meanwhile preserving surrounding normal tissues.
In this article, the authors report a case of bulky recurrent MPC in the left maxillary sinus successfully treated with radical RT and review the limited literature on the role of primary RT in sinonasal MPC management. The workflow included literature research of all index reports written in English. The search was based on the following combination of keywords: “haemangiopericytoma,” “myopericytoma,” “paranasal sinus,” “maxillary,” “head and neck region,” and “radiotherapy.” The last search data was 14th January 2022.
CASE DESCRIPTION
A 38-year-old male was evaluated due to a 3-month history of post-traumatic left maxillary haematoma and oral bleeding. The patient presented significant volume increase on the left maxillary bone region, with painful sensibility when palpated. A CT scan showed a left maxillary necrotic mass with erosion of all the bony walls of the sinus. The infiltrative mass anteriorly eroded the anterior wall of the maxillary sinus, occupying the buccal space with the swelling of the subcutaneous soft tissues and the thickening of the buccinator muscle. Below, it eroded the alveolar process with an extrinsication in the soft palate; posteriorly, it infiltrated the medial pterygoid muscle, reaching the parapharyngeal mucosa; laterally, it infiltrated the retromaxillary adipose tissue; and superiorly, it involved the pterygopalatine fossa.
As a part of the staging procedure, an incisional oral biopsy was performed. Histological analysis revealed a sinonasal haemangiopericytoma-like tumour/MPC. Microscopic examination showed an inhomogeneous cell density consisting of spindle and oval elements with a hyperdense nucleus. The neoformation was characterised by a rich vascular network of various calibre, myopericytomatosis/haemangiopercytomasis (cluster of differentiation 31-positive and cluster of differentiation 34-positive), mitotic index of 28×10 high-power field, and a Ki-67 proliferation index of 30%. Functional imaging carried out with 18-fluoro-2-deoxy-glucose PET scans confirmed the left maxillary cancer and excluded nodal or distant metastases.
To control intra-operative tumour bleeding, the patient underwent pre-surgical endovascular embolisation of left external carotid artery terminal branches. A left orbital-maxillary resection surgery was performed, with plastic reconstruction by left fibula flap, and a custom-made plate orbital grid in titanium. The histological examination of surgical specimen confirmed the finding of the biopsy. Microscopically positive margins were found (the neoplasm reached the margins of the maxillary tuberosity, the pterygoid muscle, and the resection margin of the nasal flap). In the immediate post-operative period, blood transfusions were required. The patient was afebrile, and the vital signs were normal and stable during the entire postsurgical phase.
A control CT scan 2 months after surgery showed a mass occupying the masticatory space, with longitudinal extension from the pterygoid processes to a plane passing through the uvula, with peripheral contrast enhancement and hypodense content. On physical examination, a trismus (interincisal distance at a maximum mouth opening was 10 mm) and an oral ulcer located in the hard palate were found. The patient was referred to the authors’ department (the Department of Radiation Oncology, S. Croce and Carle Teaching Hospital, Cuneo, Italy) 3 months after surgery. A repeat CT scan, performed 4 months after the initial CT scan, documented a bulky recurrence of the disease that occupied the entire maxillary sinus and emerged in the oral cavity (70 mm diameter [hl]Figure 1A[/hl]). Due to the complexity of a new surgical procedure, and the patient’s desire, a RT treatment was proposed. The patient had a CT simulation procedure in a supine position and was immobilised with thermoplastic mask. The simulation non-contrast CT scan was performed with 3 mm slice thickness axial images acquired from the vertex to the jugular notch.
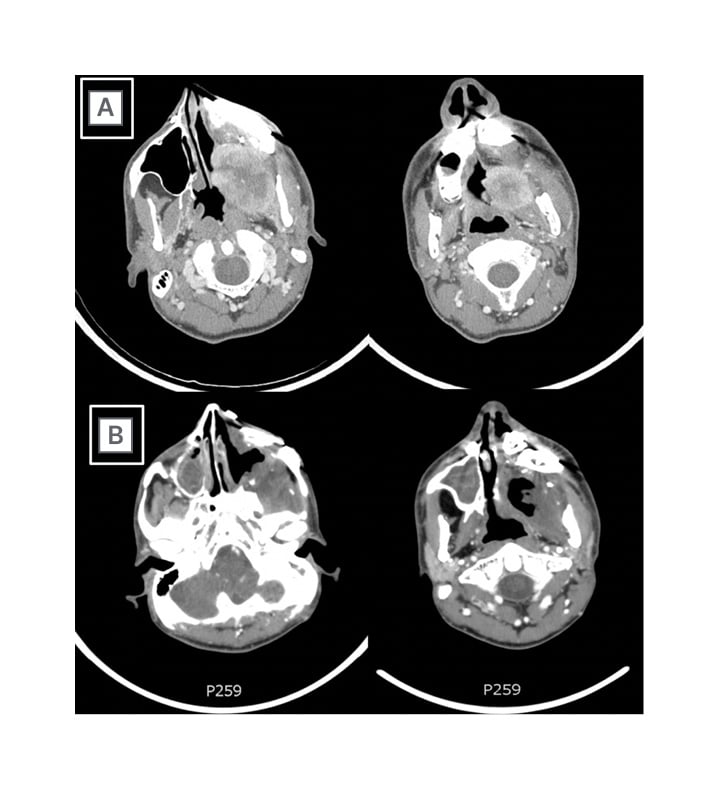
Figure 1: A) Contrast-enhanced CT scan of bulky recurrence of myopericytoma that occupied the entire maxillary sinus and emerged in the oral cavity; and B) contrast-enhanced CT scan after 3 months from the end of radiotherapy, showing the absence of disease.
An isocentre was determined within the left maxillary lesion and marked on the mask under laser guidance for daily set-up. Using a dedicated tool, diagnostic images were fused to the simulation non-contrast simulation CT scan to help delineate target volume and organs at risk. Gross tumour volume was comprised of macroscopic disease, and it was defined based on diagnostic CT information. Gross tumour volume was expanded isotropically by 5 mm to obtain subsequent clinical target volume (CTV). The latter was optimised to include flaps and reconstructive plates, and to avoid organs at risk. The planning target volume (PTV) was defined by 3 mm isotropic expansion around CTV. The prescribed dose to the disease was 67.5 Gy/30 fractions. RT was administrated daily, on 5 consecutive days per week. To reduce the dose to critical structures, an intensity-modulated RT with volumetric-modulated arc therapy approach was performed. Dose-volume constraints for normal tissues and PTV dose coverage were reported in Table 1.
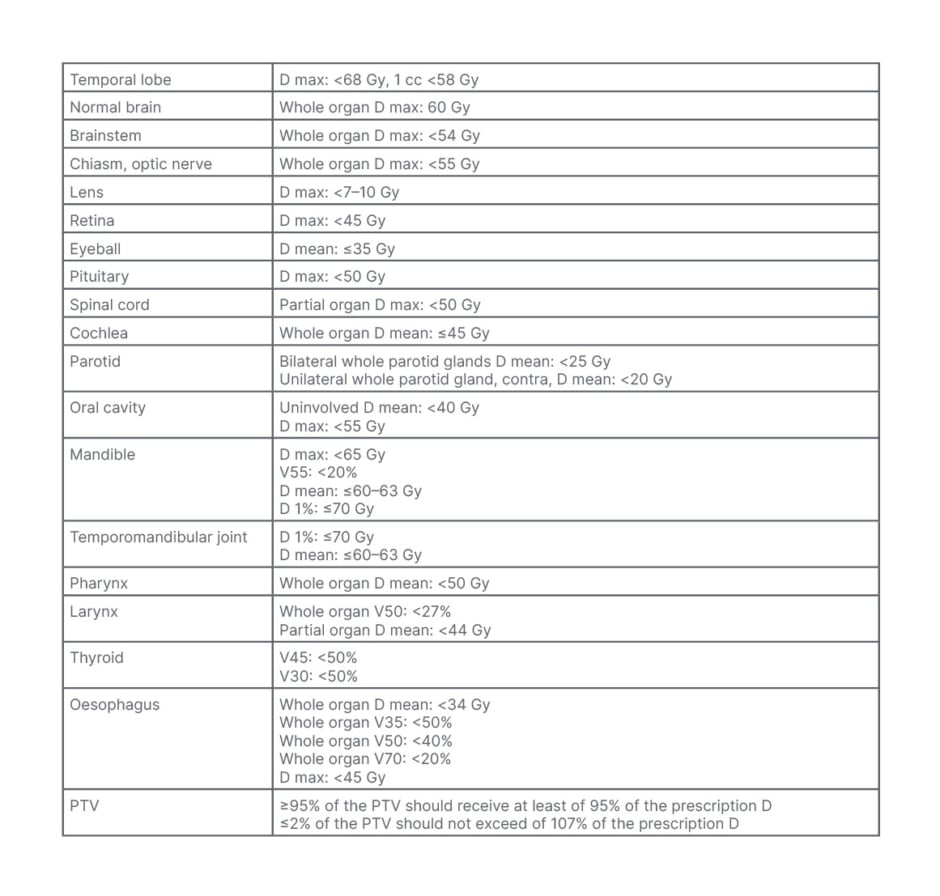
Table 1: Dose-volume constraints for normal tissues and planning target volume dose coverage.
D: dose; max: maximum; PTV: planning target volume; V: volume
To verify daily set-up and inter-fraction organ motion, image-guided RT was employed, with an automatic matching of cone-beam CT images acquired prior to each fraction delivery and reference planning CT (Figure 2). The treatment was delivered on a TrueBeam machine (Varian Medical Systems, Palo Alto, California, USA), using a multi-leaf collimator with 60 leave pairs. The patient experienced acute Grade (G) 2 skin toxicity and G1 oral mucositis, according to the Common Terminology Criteria for Adverse Events, version 5 classification.6
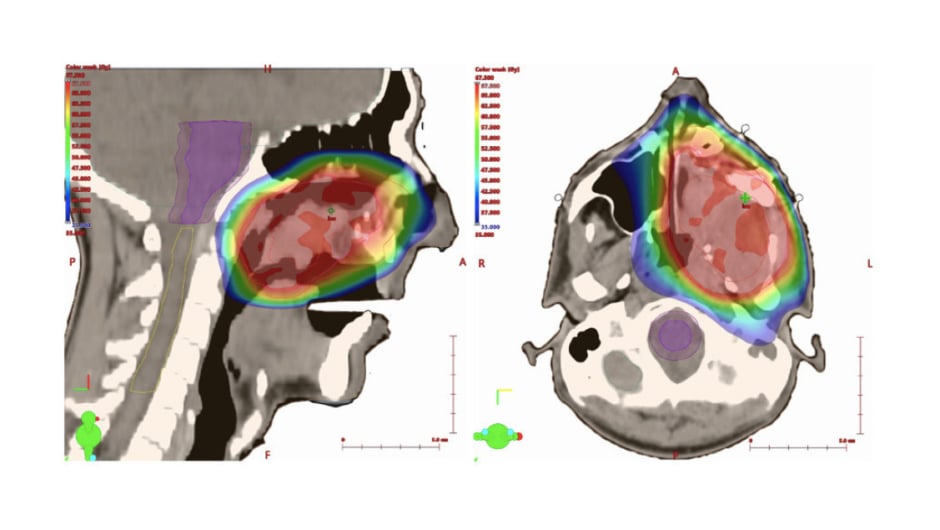
Figure 2: Intensity modulated radiotherapy planning of sinonasal myopericytoma.
During follow-up, a CT scan acquired at 1 month after the end of RT showed a residual tissue without contrast enhancement. The next CT scan 3 months after the end of the treatment confirmed the absence of disease (Figure 1B). The tissue defect, caused by the disease response, formed a fistula between the oral cavity and maxillary sinus. It was not possible to treat the fistula by the placement of an obturator prosthesis due to the pre-existing trismus, which further worsened after RT (the interincisal distance was 3 mm). Residual left maxillary region alterations were treated with lipofilling procedures with aesthetic purposes. Long-term toxicities, identified 5 years after the end of radiotherapy, were trismus G3, dysgeusia G2, dysphagia G2, dry mouth G1, skin induration G2 (according to the Common Terminology Criteria for Adverse Events, version 5 classification).6
Currently, patient is disease-free; however, their quality of speech is reduced. Through the extraction of some teeth (teeth numbered 23, 24, 25, 33, and 34 were removed), soft oral feeding is allowed. The skin above the titanium plates remains thin but trophic.
DISCUSSION
In the case presented by the authors, the patient with bulky recurrent sinonasal MPC achieved a persistent complete response after definitive RT. The patient is still alive and recurrence-free 54 months from the start of treatment. Overall, RT was well tolerated, with high compliance and mild sequelae. Given the speed of growth, a moderately hypo-fractionated RT schedule was chosen, employing 2.25 Gy per fraction to a total dose of 67.5 Gy. The treatment volume included the relapse site, flaps, and reconstructive plates. For the complexity of the volume and the proximity to some critical structures (orbit, optic nerve, temporomandibular joint, masticatory space, and skull base), an intensity-modulated RT-image-guided RT technique was chosen to minimise the CTV-PTV expansion margins at 3 mm.
Clinical data regarding the use of definitive RT as primary treatment modality in sinonasal MPC are scarce. A comprehensive and evidence-based analysis is difficult. In fact, to the best of the authors’ knowledge, only one other case of MPC was successfully treated in recent years with primary RT and has been described in the literature.7 In that clinical case, a 78-year-old female presented with an enlarged MPC in the soft palate. The patient refused surgical approach and, thus, RT was proposed. A total dose of 66 Gy in daily fractions of 2 Gy was prescribed. A remarkable tumour regression was recorded and there was no sign of tumour growth 4 months after treatment.
While the role of primary RT is pending, several studies addressed the role of adjuvant RT.2,8-10 For sure, surgery with wide margins is the standard of care for patients with MPC. A systematic review of individual cases of sinonasal MPC, including 97 articles published between 1950–2009, pointed out a complete excision is essential to minimise tumour recurrence, and RT may increase the disease-free survival and decrease the rate of recurrence when complete resection is not possible.2 Recently, a retrospective study investigated the role of RT to reduce the risk of recurrence after surgery in the framework of the Rare Cancer Network (RCN), in a cohort of patients diagnosed with extracranial MPC. The median RT dose was 60.0 Gy (range: 45.0–68.4 Gy) in 1.6–2.2 Gy fractions. An improvement of prognosis in terms of local control rates and disease-free survival was observed, especially in patients with extremities/superficial trunk tumour locations.9 Lastly, a multi-institutional retrospective study (KROG 18-11) evaluated the role of postoperative RT in 133 patients with histologically confirmed intracranial solitary fibrous tumours/MPC.10 The study supported the role of post-operative RT in disease control of intracranial MPC, irrespective of the surgical extent and G. For local control, post-operative RT needs to enclose the residual tumour or surgical cavity with sufficient margin for the target volume.
On the other hand, registry analysis and retrospective series supported the absence of clinical benefit of adjuvant RT in patients with MPS. A Surveillance, Epidemiology, and End Results (SEER) registry population-based analysis of 1,243 patients with MPC, treated from 1975–2016, highlighted that surgery remains the standard of care to assure significant overall survival and cancer-free survival rates.11 Surgery plus adjuvant RT had a similar survival effect compared with surgery alone (overall survival: p=0.900; cancer-free survival: p=0.156). Likewise, a systematic review of sinonasal MPC management (128 cases collected from 56 articles, consisting of case reports and series) confirmed surgical excision as mainstay of treatment, limiting the role of RT both in primary and adjuvant setting.8 In their meta-analysis of 116 primary head and neck MPC cases, Wushou et al.12 confirmed that surgery plus adjuvant RT was not superior to surgery alone, and concluded that surgical resection should be the first step in MPC management. It is tempting to consider that RT has a marginal role, but important limitations may have led to underestimations in the clinical efficacy of RT. All studies are retrospective and the lack of tumour- and RT-related details such as body site of origin and RT protocols and technique, which may produce inconsistent conclusions.
At this point, classification of MPC radiosensitivity remains the principal question. Although it is traditionally believed that MPC is resistant to ionising radiation, its radiosensitivity has been somewhat controversial. Several reports have shown the presence of tumour features that may confer sensitive response to radiation. High cellular metabolism, rapid cellular growth, and increased or unstable vascularity are recognised predictors of a good response to radiation; however, other factors such as the presence of necrosis and host immune responses are not.13 At present, there are no known biomarkers predictive of radiosensitivity. A dose-response relationship has been reported for MPC in older series.4,14 Mira et al.4 published a study with the purpose to analyse the response of MPC to RT in 11 patients with MPC treated at Memorial Hospital (Memorial Sloan-Kettering Cancer Center [MSKCC], New York City, New York, USA). Dose and tumour size were found to be the main factors influencing response.4 This was consistent with a report by Staples et al.14 who showed that a total dose greater than 55 Gy improved local control in patients with MPC.
Overall, literature data are extremely poor to provide any firm recommendation. In the absence of robust evidence and guidelines, each MPC case should be discussed in a multidisciplinary tumour board. High-dose definitive RT should be considered for those sinonasal MPC cases unresectable or unfit for surgery.
CONCLUSION
The role of definitive RT in sinonasal MPC remains largely unknown. This case report clearly indicates the value of high-dose definitive RT using modern technique in the management of patients with MPC. The authors suggest a multi-institutional registry for this rare entity to help guide treatment.







