Chairperson: Angelo Dei Tos1,2
Speakers: Rosario García-Campelo3, Wilko Weichert4, Angelo Dei Tos
1. Department of Pathology, Azienda Ospedaliera Università di Padova, Padua, Italy
2. Department of Medicine, Università di Padova, Italy
3. Medical Oncology, A Coruña University Hospital, Spain
4. Institute of Pathology, School of Medicine, Technical University of Munich, Germany
Disclosure: Dei Tos has received fees for advisory services from Bayer, GlaxoSmithKline, Novartis Oncology, and Roche; and educational grants from PharmaMar. García-Campelo has received fees for consultancy or advisory service or lectureships from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Meyer Squibb, Janssen, Lilly, Novartis, MSD, Roche, Sanofi, Pfizer, and Takeda. Weichert has received fees for advisory service or lectureships from ADC Therapeutics, Agilent, Amgen, Astellas, AstraZeneca, Bayer, Boehringer, Bristol Myers Squibb, Eisai, GlaxoSmithKline, Illumina, Janssen, Lilly, Merck, Molecular Health, MSD, Novartis, Pfizer, Roche, Siemens, and Takeda; and fees for research funding from AstraZeneca, Bristol-Myers Squibb, MSD, and Roche.
Acknowledgements: Writing assistance was provided by Bronwyn Boyes, London, UK.
Support: The publication of this article was funded by Novartis. The views and opinions expressed as those of the authors and not necessarily Novartis.
Citation: EMJ Oncol. 2022;10[Suppl 1]:2-10
Session Summary
Lung cancer has a devastating global impact, claiming almost 2 million lives in 2020.1 Despite advances in treatment, lung cancer remains the world’s leading cause of cancer-related morbidity and mortality.2 Non-small cell lung cancer (NSCLC) is the most common tumour type, accounting for around 85% of lung cancers,3 and the majority of patients present with advanced disease at diagnosis.4 One of the most significant advances in the treatment of NSCLC has been the introduction of personalised medicine through the identification and targeting of driver mutations.4 Several molecular drivers have been identified that represent strong predictive biomarkers and serve as therapeutic targets. Testing for key biomarkers is now considered mandatory in many countries.4 Lung cancer provides the best model for molecular prediction and personalised medicine which can be shared with other cancers, including breast cancer.
The Role of MET Inhibitors in the Treatment of Non-small Cell Lung Cancer: Clinical Benefits and Practical Considerations of Genomic Testing
Rosario García-Campelo
García-Campelo started her presentation by stating that the field of oncology is experiencing an extraordinary moment. She believes that three aspects define the situation: personalised medicine; innovation; and, for the first time, there is a possibility of a cure in some specific advanced NSCLC patients.
In 2020, Howlader et al.5 showed, for the very first time, a decrease in the annual numbers of NSCLC mortality. This decrease could be due to improved screening and educational programmes, but also as a result of personalised medicine and the availability of innovative therapeutic agents. NSCLC is one of the best examples of personalised medicine, with a growing list of genetic alterations and a subsequently increasing number of targeted therapeutic agents in specific subsets of NSCLC.6 It is useful to screen for even rare alterations, as it might change the lives of patients with NSCLC.
The European Society for Medical Oncology (ESMO) guidelines recommend mandatory systematic molecular testing for EGFR, ALK, ROS1, BRAF, NTRK1, and programmed death-ligand 1 (PD-L1). In addition, the ESMO guidelines also suggest testing for evolving targets or biomarkers such as RET, HER2, KRAS, and MET receptor tyrosine kinase alterations (Figure 1).4 These should be considered mandatory testing very soon, according to García-Campelo.
There are different types of MET alterations found in NSCLC. The reported prevalence of overexpression of MET in NSCLC varies from 25–75% and has not proven to be a good predictive biomarker for response to a specific targeted therapy so far.7METex14 skipping and MET amplification have been identified as oncogenic drivers in 3%,8-10 and 3–7%7 of patients with NSCLC, respectively. Specific MET alterations are associated with distinct patient characteristics. METex14 skipping usually occurs in older (≥70 years) females and non-smokers.10,11MET amplifications typically occurs in older (≥70 years) males and smokers.8,12,13 While EGFR and ALK mutations are more frequently found in young patients who never smoked, García-Campelo stressed the importance of remembering that MET alterations can be detected in a broader population. Aberrant activation of the MET pathway through different alterations in the MET gene promotes tumour growth and metastasis. This is associated with aggressive disease, poor prognosis and can drive resistance to other cancer therapies.10,14 Both MET skipping and MET amplifications can co-exist.8,12 In fact, MET amplifications co-occurs in approximately 12% of patients with METex14 skipping mutations.12,15MET alterations can also be a co-driver with other alterations and have been described to develop a resistance mechanism in up to 15% of patients with EGFR, ALK, or ROS1 mutations treated with tyrosine kinase inhibitors (TKI).14,16,17
With the introduction of new drugs, including TKIs and monoclonal antibodies, the question is, should patients with MET alterations be treated with a MET directed targeted agent? Awad et al. demonstrated, in a multicentre retrospective analysis of 148 patients with METex14 NSCLC, that treatment with a MET inhibitor was associated with an improvement in overall survival (OS). In patients who never received a MET inhibitor, the median OS was 8.1 months, and in those who received at least one MET inhibitor, the median OS was 24.6 months.18 Despite the limitations of a retrospective study, García-Campelo believes that these improvements in OS make a huge difference for patients. García-Campelo then discussed the clinical activity of key MET TKIs from recent prospective clinical trials in treatment-naïve and previously treated patients with METex14 skipping NSCLC.19-23 MET multi-kinase inhibitors such as crizotinib (evaluated in the PROFILE 1001 trial)19 and, more recently, selective MET inhibitors such as capmatinib (GEOMETRY trial)20,21 tepotinib (VISION trial),22 and savolitinib (Lu et al.)23 have demonstrated clinical efficacy and safety in pre-treated and treatment-naïve patients with METex14 NSCLC. These studies demonstrated significant clinical activity (including overall response rate, progression-free survival, and OS data) in those treated with the METex14 skipping mutation inhibitors. García-Campelo further explained that there is, overall, a better response in treatment-naïve patients with METex14 NSCLC.
For patients with a MET amplification, the activity of MET TKIs appears to depend on the degree of amplification. A current consensus on the optimal diagnostic cut off for MET amplification is, however, lacking. The results of MET-directed targeted therapy in patients with MET amplification is modest when compared to those with METex14 skipping mutation. This highlights the need for further research into which patients with MET amplification would most benefit from targeted therapy.24
As previously mentioned, MET can also occur as a secondary alteration or resistance mechanism in response to therapy in specific populations like EGFR-mutated NSCLC. García-Campelo shared two interesting clinical trials. The TATTON25 and INSIGHT26 studies highlight that combination of EGFR and MET inhibitors might be a potential treatment option for patients with MET-driven resistance to EGFR TKIs.
The CHRYSALIS trial, evaluating the combination of amivantamab and lazertinib in patients with EGFR-mutant NSCLC after progression on osimertinib showed that efficacy depends on whether the resistance to EGFR therapy is driven by MET, EGFR, neither, or both.27,28 Other ongoing trials investigating resistance mechanisms include the ORCHARD29 andSAVANNAH30 trials, additional MET targeting agents in development include Sym01531,32 and telisotuzumab vedotin.29
García-Campelo feels that it is important to understand or anticipate the molecular mechanism of resistance as soon as possible and incorporate it into a dynamic treatment strategy.33 Recondo et al. showed that genomic on-target and bypass mechanisms of resistance were frequently found in the setting of resistance to MET TKI.34MET-dependent resistance includes single and polyclonal kinase domain mutations in frequent hotspots (D1228, Y1230, and L1195) and high levels of MET amplification. Genomic bypass mechanisms of resistance involve recurrent gene amplification in EGFR, HER2, HER3, and MAPK pathway genes (KRAS/BRAF) and KRAS mutations. Depending on the type of resistance, treatment strategies like sequential MET TKI for on-target resistance, and EGFR-MET, or MET-MEK dual combinations for bypass activation should be explored in future clinical studies.34
García-Campelo then discussed the role of PD-L1 in METex14 NSCLC. Although PD-L1 expression might be higher in METex14 NSCLC, tumour mutational burden distribution was significantly lower compared with the wild-type population.35 Several retrospective studies including the Memorial Sloan Kettering Cancer Center (MSKCC) cohort,35 the IMMUNOTARGET registry,36 and the GFPC 01-201837 trials showed only modest efficacy results with immune checkpoint inhibitors in METex14 NSCLC. In contrast, patients with high levels of MET amplifications seem to benefit more from immunotherapy.38 García-Campelo surmised that it is imperative to better understand the high genetic heterogeneity of MET-aberrant NSCLC and its impact on immunotherapy outcomes.
Genomic Testing in Non-small Cell Lung Cancer: Current Challenges and New Approaches with a Focus on MET-Testing
Wilko Weichert
Weichert started his talk with an overview of the development and importance of precision medicine and molecular testing. The NSCLC treatment landscape has changed tremendously in the last decade, with discoveries made in molecular biology. Only 15 years ago, lung cancer was classified mainly by its histology. Today, there are a plethora of entities defined by molecular alterations, with different treatment approaches for each type of tumour. This has led to fundamental changes in treatment algorithms. Historically, everyone received platinum-based chemotherapeutic agents, which brought no survival advantages over decades. Today, before initiating treatment, molecular profiles are used to target specific molecules and define sub-entities of NSCLC (Figure 1) .4
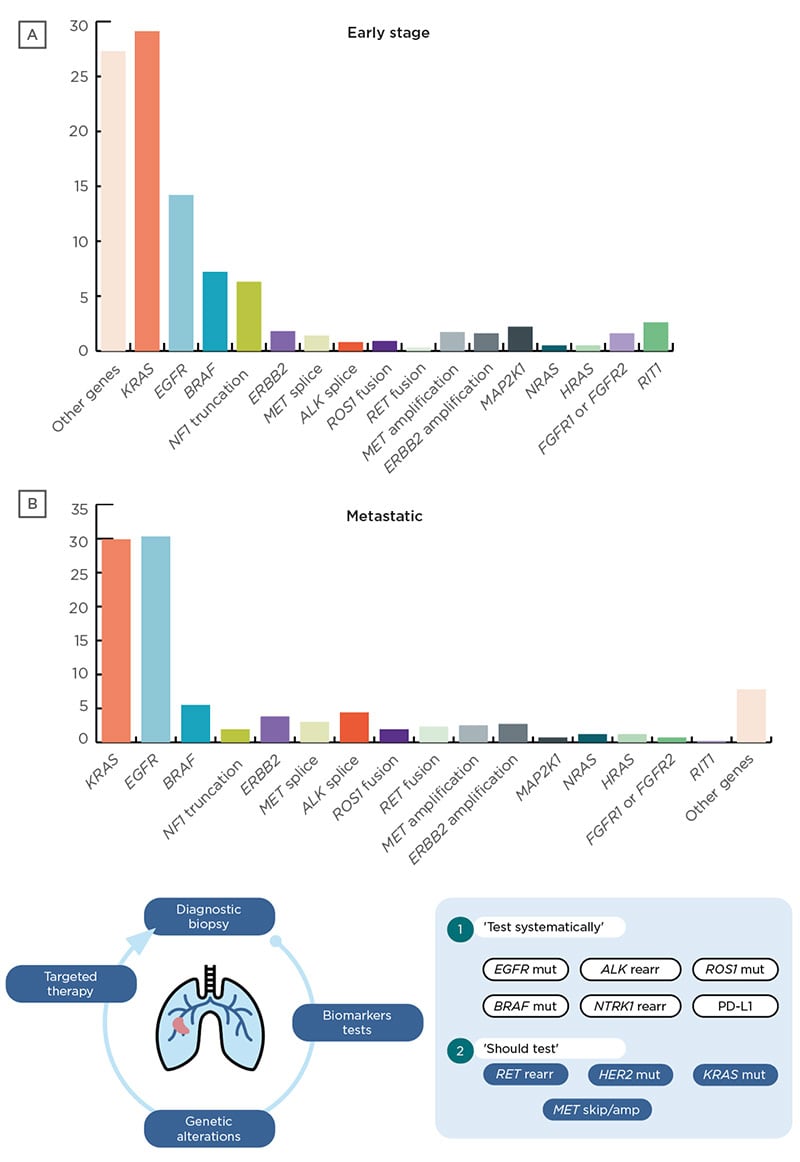
Figure 1: Genomic alterations in non-small cell lung cancer and guideline testing recommendations.4,6
Amp: amplification; mut: mutation; rearr: rearrangement.
Weichert then reviewed the sequencing technology that led to this changing treatment landscape. He discussed that most, but not all, biomarkers can be detected by sequencing.39,40 Sequencing technologies can be used to test for single alterations, for panels of genes or to screen genome wide, detecting different alteration such as base substitutions, genomic rearrangements, copy number changes, and insertions or deletions.41
Specific targeted agents are now available for several genetic alterations. KRAS alterations, which can occur in approximately 29% of patients with NSCLC,6 were previously considered as undruggable. However, recent therapies such as sotorasib and adagrasib have shown good efficacy for patients with a KRAS G12C mutation in the CodeBreak 10042,43 and KRYSTAL-144,45 Phase II studies, respectively. These trials also highlight the trend of considering broader molecular testing in order to detect other aberrations that might predict outcomes and offer additional therapeutic targets for future combinatorial approaches.
EGFR mutations were first discovered more than a decade ago, occurring in approximately 14.2% and 30.3% of patients with early stage and metastatic NSCLC, respectively.6 Recent research is investigating rare mutations like Exon 20, resistance mechanisms to EGFR directed therapy, and the role of targeted adjuvant treatment in patients with EGFR mutated tumours.46 Similarly, BRAF and ERBB2 mutations, which were previously considered untreatable, now have therapeutic options available or in development. Treatment options for ALK alterations and translocations have evolved tremendously since the first approval in 2012, with eight regulatory approvals in the last 8 years, with second- and third-generation options to target resistance.
Another rapidly evolving area of research is in METex14 skipping mutations and MET amplification.8-10,11,12,47,48 Among the methods available for detecting METex14 are reverse transcription-PCR and RNA- or DNA-based sequencing methods.49-52 Each of these methods have potential shortcomings such as mutations in the primer binding site, which can affect primer-binding for PCR-based methods. Another example are mutations detected by sequencing that have not been described as ‘skipping initiating’.49-52 A study comparing diagnostic assays for METex14 found that RNA-based next-generation sequencing (NGS) should be the assay of choice as a multiplex test. Sanger sequencing can detect METex14 with 100% specificity but a sensitivity of only 61.5%. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was found to be sensitive (100%) and specific (97.4%) and may be appropriate for screening METex14 as a single gene testing.53
Besides detecting oncogenic drivers that can be treated by targeted therapies, it becomes increasingly important to understand the genomic alterations on a broader scale to support treatment decisions and predict responses. In addition, detailed knowledge of prior therapeutic exposures is critical for accurate interpretation and understanding of the impact of mutational processes.6
Liquid biopsy testing from blood samples can also be useful, with the mainstay being the analysis of circulating tumour DNA. Liquid biopsy may be used to complement tissue biopsy, when tumour tissue is scarce or unavailable, or when a significant delay in obtaining tissue is anticipated.49 Liquid biopsy might be especially important in the future for MET testing in scenarios of molecular resistance (e.g., in response to EGFR directed therapy) to ensure appropriate treatment.54
Weichert concluded that broad multigene testing in lung cancer is mandatory, with new trends of earlier and more frequent testing, especially for resistance. Quality assurance is mandatory (ensuring that what is used is reliable), and for METex14-specific mutations, clinicians should use DNA- or RNA-based NGS methods in conjunction.
Genomic Testing in Breast Cancer: Lessons Learned from Non-small Cell Lung Cancer and Future Perspectives with Focus on PIK3CA-Testing
Angelo Dei Tos
Predictive biomarkers have been utilised in breast cancer for several years, including oestrogen receptor, progesterone, human epidermal growth factor receptor 2 (HER2), Oncotype DX, PD-L1, and PIK3CA.55 Dei Tos believes that as predictive biomarkers have become integral in the use of targeted therapies to treat lung cancer, other fields of cancer should look to this field for guidance and learnings.
The phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/ mechanistic target of rapamycin (mTOR) pathway is an important intracellular signalling pathway for protein synthesis, cellular proliferation and survival, glucose metabolism, apoptosis, DNA repair, and genome stability.56 Any aberration of this pathway will strongly influence the fate of the cells and particularly carcinogenesis. Mutations in the p110a catalytic subunit of PI3K (PIK3CA) have been identified in gastric cancers (18.0%), colorectal cancers (15.0%), breast cancer (20.0–50.0%), and head and neck squamous cell cancers (30.5%). Mutations in PI3K are not the only method of altering this pathway. In fact, genomic amplification occurs more frequently in NSCLC, loss of phosphatase and tensin homologue can occur in 20–40% of colorectal cancers, mTOR activation can occur in 40% of bladder and prostate cancers, and increased AKT1 activity can occur in 40% of breast cancer and ovarian cancers and 50% of prostate cancers.57
Several therapies including PI3K, AKT, mTOR, and dual PI3K/mTOR inhibitors are currently available or in clinical development.58 Furthermore, there are three classes of PI3K inhibitors. The pan-PI3K inhibitors, which inhibits all four catalytic isoforms of Class I PI3K, have broad inhibitory potential in several tumours, albeit with higher risks of side effects and toxicities. The isoform specific PI3K inhibitors, which selectively inhibit specific PI3Ks, enabling precise targeting, thereby reducing off-target toxicities, require careful patient selection. Examples include idelalisib, a PI3Kδ inhibitor, for the treatment of relapsed chronic lymphocytic leukaemia and in combination with rituximab for the treatment of relapsed follicular B cell non-Hodgkin lymphoma (and alpelisib, a PI3Kα-specific inhibitor that is approved for use in combination with endocrine therapy fulvestrant for the treatment of hormone-positive (HR+), HER2-negative (HER2-), PIK3CA-mutated, advanced or metastatic breast cancer. The dual PI3K/mTOR inhibitors target both PI3K and mTOR signalling. To date, no dual PI3K/mTOR inhibitor has received approval for treatment of any cancer.59,60
The drug-related toxicities from small-molecule PI3K inhibitors depend on their PI3K isozyme specificity. Common adverse effects associated with PI3Kα inhibitors include rash and hyperglycaemia; for PI3Kδ inhibitors, gastrointestinal adverse effects, transaminitis, and myelosuppression; and for pan-PI3K inhibitors dose-dependent toxicities include fatigue, diarrhoea, rash, and hyperglycaemia.60
Dei Tos then reviewed the molecular landscape of breast cancer, where 71% of patients have HR+/HER2-, 12% have HR+/HER2+, 12% have HR-/HER2-, and 5% have HR-/HER2+. Furthermore, approximately 40% of people with HR+/HER2- breast cancer have a PIK3CA mutation.61 The PIK3CA mutational landscape is relatively complex; heterogeneous and mutations occur in multiple domains with different effects on PI3K activity and sensitivity to PI3K inhibitors.55 In addition, Razavi et al.62 identified loss-of-function PTEN mutations in 25% of patients with resistance to alpelisib plus aromatase inhibitor.This further complicates the landscape when deciding on the best treatment options for patients with breast cancer. Therefore, there exists a clinical need for a better understanding of the mutational landscape of PI3K and the interpretation of clinical studies as well as its translation to daily clinical practice.
Sanger sequencing is a gold standard of PIK3CA screening, with 99.99% accuracy but only 15–20% sensitivity. Furthermore, it is highly time consuming and takes approximately 2 days for results of one specific sequence.63,64 Droplet digital PCR is emerging as the most precise and sensitive digital PCR solution for a wide variety of applications, with a sensitivity of one mutant allele among 180,000 wild-type molecules, and a fast turnaround of approximately 4 hours to obtain a result.65 In real-life molecular pathology and especially for PIK3CA mutations, the methodology most often used is a qRT-PCR-based approach. The only difference between the many qRT-PCR approaches is the number of mutations detected. Martínez-Sáez et al. showed that PIK3CA mutations in breast cancer are highly heterogenous, and the currently validated and U.S. Food and Drug Administration (FDA)-approved therascreen® companion diagnostic test might not capture up to 20% of patients with PIK3CA mutations. This finding highlights an urgent need to better identify patients that may benefit from alpelisib or other PI3K inhibitors.66
Dei Tos then reviewed whether the use of NGS is justified for all patients with breast cancer, or if it should only be limited to selected cases or for patients discussed in molecular tumour boards. Also, in the era of liquid biopsy, tumour tissue is not the only option for molecular testing. Although less efficient, clinicians should also consider non-invasive liquid biopsy, if necessary, especially in cases where tumour tissue is not available. Patients with a negative liquid biopsy result should, however, undergo a second liquid biopsy test or tissue biopsy to avoid false-negative results. Tissue samples from initial diagnosis (primary tumour) are a reliable source for PIK3CA-testing, but at the same time only provide a snapshot of tumour development. Therefore, if clinically feasible, clinicians should also consider molecular analysis of the metastatic sites as cancers evolve over time. The scientific community seem to promote the use of NGS technology; however, it is imperative for clinicians to select the most appropriate and practical testing method for their patients and practice, considering all the available techniques.49-52
Dei Tos summarised his presentation with the following: the PI3K/AKT/mTOR pathway is crucial in regulating several key cellular processes; the PI3K/AKT/mTOR pathway plays a pivotal role in cancer; the PI3K/AKT/mTOR pathway is a potential target for molecular cancer therapy; the use of PI3K inhibitors requires careful patient selection through molecular analysis of PIK3CA; breast cancer takes advantage from the experience developed within the lung cancer community; and lung cancer has been the best model for molecular prediction (a lesson that has also been learned for breast cancer) a field now being ready to implement PI3K mutational analysis to help the afore mentioned 40% of patients with breast cancer to get the best available therapeutic options.








