Meeting Summary
Prof D’Amore opened the symposium by highlighting that management of patients with relapsed or refractory aggressive B cell non-Hodgkin lymphoma (NHL) remains an unmet clinical need because of its poor prognosis and the lack of effective therapeutic options. He proceeded to introduce pixantrone, the first approved single-agent treatment for the management of aggressive NHL in the third or fourth lines. Dr Lugtenburg then outlined the current treatment landscape for diffuse large B cell lymphoma (DLBCL). Dr Pettengell presented clinical evidence from the PIX301 study, explaining the clinical evidence behind the regulatory approvals for the use of pixantrone in relapsed or refractory aggressive NHL as well as discussing the mechanism of action of pixantrone. Prof Zinzani discussed the use of pixantrone as a new therapeutic option in clinical practice, and was followed by Prof Cordoba, who presented two clinical cases of patients treated with pixantrone. The symposium concluded with a panel discussion.
Introduction
Professor Francesco D’Amore
Pixantrone is the first single-agent treatment for the management of aggressive NHL in the third or fourth lines approved by the European Medicines Agency (EMA)1 on the basis of data from the PIX301 study. It is indicated for the treatment of adult patients with multiple relapsed or refractory aggressive NHL and this recommendation is reflected in national/international treatment guidelines such as the European Society of Medical Oncology (ESMO)2 and National Institute for Health and Care Excellence (NICE) guidance in the UK.3 The use of this new treatment option in clinical practice is currently being established.
Current Treatment Landscape of Diffuse Large B Cell Lymphoma
Doctor Pieternella Lugtenburg
Epidemiology and Prognosis
The majority (about 85%) of NHLs arise from B-lymphocytes; with DLBCL being the most common subtype (37%).4 DLBCL can present de novo, or as a transformation from a more indolent form of lymphoma.5 The incidence of DLBCL varies across the world, ranging from 3.8 per 100,000 inhabitants in Europe6 to 7 per 100,000 in the USA. It is mainly a disease of the middle-aged and elderly, with a median age at presentation of 64 years. Known risk factors for DLBCL include a family history of haematological malignancies, autoimmune diseases (such as Sjögren’s syndrome), and certain viral infections like HIV.7 Immunosuppression is also a well-known risk factor for the development of DLBCL.
DLBCL is a curable disease; data from the French Cancer Registry Population have shown a favourable prognosis for DLBCL. Even though elderly patients have a poorer prognosis than young patients, the prognosis for all age groups over the last decade has improved significantly,8 primarily due to the introduction of rituximab, a monoclonal antibody that targets the CD20 antigen expressed on almost all B cell lymphomas.9
DLBCL is a heterogeneous disease and, as such, not all patients have the same prognosis; in the clinic, the International Prognostic Index (IPI) score is used to determine the prognosis. The IPI score is determined by five different negative prognostic factors related to the patient and disease (age, performance status, lactate dehydrogenase levels, stage of disease, and extranodal lesions).10 Gene-expression profile studies have also revealed two important molecular subtypes of DLBCL according to the cell-of-origin: germinal-centre B cell-like (GCB) DLBCL and activated B cell-like (ABC) DLBCL. Patients with the ABC subtype have been shown to have a worse outcome compared with those with the GCB subtype,11 and therefore, there is a high unmet medical need in this subgroup of patients.
Treatment Algorithm for Aggressive Non-Hodgkin Lymphoma
Figure 1 depicts a treatment algorithm for treatment of aggressive NHL.12 According to the ESMO guidelines, R-CHOP (rituximab with cyclophosphamide, doxorubicin [hydroxydaunomycin], vincristine, and prednisolone) or R-CHOP-like therapy are recommended for first-line treatment of aggressive NHL for both young and elderly fit patients.12 Patients with a high IPI score who are at a high risk of relapse could be given a more intensive regimen, such as initial high-dose chemotherapy (HDCT) followed by autologous stem-cell transplantation (ASCT). Doxorubicin can be substituted with other drugs (e.g. gemcitabine, etoposide, or liposomal doxorubicin) in patients who are unfit or frail. With R-CHOP treatment, between 50% and 60% of patients are cured, 30–40% relapse, and 10% have refractory disease.13 Most relapses occur within the first 2 years of initiation of therapy, and are usually symptomatic;14 this patient population with relapsed/refractory (RR)-DLCBL disease is very heterogeneous and has a poor life expectancy.
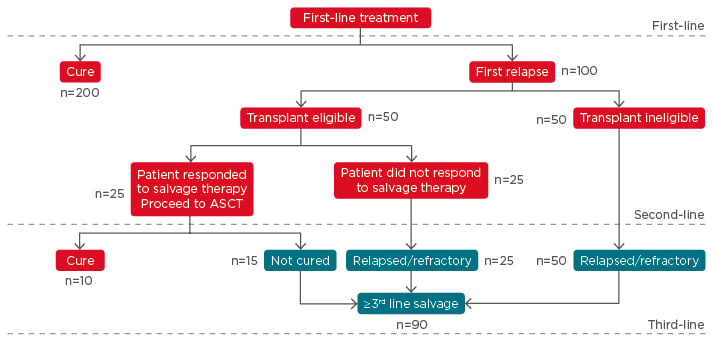
Figure 1: A treatment algorithm for aggressive B cell non-Hodgkin lymphoma.*
*Based on 300 patients diagnosed with diffuse large B cell lymphoma.
ASCT: autologous stem cell transplantation.
Adapted from Friedberg 2011.12
Following relapse, the eligibility of patients with RR-DLBCL for HDCT followed by ASCT can be assessed using criteria from various organisations, such as the American Society for Blood and Marrow Transplantation (ASBMT)15 and Grupo Español de Linfomas/Trasplante Autólogo de Médula Ósea (GEL-TAMO) experience.16 Additional considerations include performance status of the patient and organ (cardiac, pulmonary, liver, and kidney) function.
Transplant-eligible patients should be treated with salvage chemotherapy regimens (i.e. rituximab, cisplatin, cytarabine, dexamethasone [R-DHAP]; rituximab, ifosfamide, carboplatin, etoposide [R-ICE]; rituximab, cisplatin, gemcitabine, dexamethasone [R-GDP]); if patients are responsive, this will be followed by HDCT and subsequent ASCT.2 Allogeneic stem-cell transplantation should be considered if patients relapse after rituximab-HDCT with ASCT. The role of ASCT following HDCT in relapsed DLBCL has previously been established as standard-of-care in the PARMA trial.5,17
To date, only two randomised controlled trials in RR-DLBCL have been carried out: the CORAL study evaluating R-DHAP versus R-ICE; and another comparing R-DHAP and R-GDP. There was no difference in efficacy; however, differences in toxicity profiles were observed among the various treatment regimens. Although the best therapy for second-line treatment has not been established, it was suggested that clinicians should prescribe the treatment that they are most familiar with, while evaluating the comorbidities of the patient balanced against the toxicity of the chosen regimen. In the CORAL study, subanalyses of event-free survival showed that patients with early relapse (<12 months after diagnosis) had a significantly better survival rate if they had not received prior rituximab;18 these results indicate that patients treated with rituximab in the first line cannot be salvaged with the current salvage therapies and therefore have a high unmet clinical need. In the Bio-CORAL study, which evaluated R-DHAP versus R-ICE, patients with GCB DLBCL responded significantly better to R-DHAP compared to patients with non-GCB subtypes.19 Thus, cell-of-origin remains a major and independent factor in RR-DLBCL.
Transplant-ineligible patients generally receive palliative treatment with platinum and/or gemcitabine-based regimens or are treated with novel drugs in clinical trials.2 R-DHAP or R-ICE regimens are generally not considered because they are too toxic. The most frequently used combination regimens are those that contain gemcitabine, oxaliplatin, lenalidomide, and/or bendamustine. Rituximab is frequently added to salvage regimens to improve outcomes.20 In the Netherlands, the PECC (prednisone, etoposide, chlorambucil, and lomustine) regimen is utilised; particularly for elderly patients. The regimen has a low toxicity profile and results in an overall response rate (ORR) in >50% of the patients and complete remission (duration <12 months) in half of these patients.21
With regards to third-line treatment following relapse/progress of disease (Figure 1), the ESMO guidelines recommend allogeneic stem cell transplantation (SCT) as an option (for transplant-eligible patients).2 The eligibility/feasibility for allogeneic SCT has improved in the last decade, mainly due to the use of the reduced intensity conditioning regimen. Encouraging cure rates (40%) have been observed, though few patients are eligible for a second transplant.22 Apart from allogeneic SCT, other treatment options include palliative care and drugs from clinical trials. A number of single-agent therapies are also available for third- or fourth-line treatment, of which pixantrone seems promising. In conclusion, 40% of patients with DLBCL who fail first-line R-CHOP treatment have a dismal outlook, and novel therapies are warranted for this patient population.
Relapsed/Refractory Aggressive B Cell Non-Hodgkin Lymphoma: What Can We Expect in Third- and Fourth-line Treatments?
Doctor Ruth Pettengell
Depending on IPI risk factor at presentation, between 5% and 50% of patients with DLBCL will relapse, with the majority (96%) relapsing within the first year. Of the 30% of patients eligible for intensive salvage therapy, only 50% actually receive a transplant, with 40% subsequently progressing within the first year. These patients, together with those who fail/respond poorly to salvage induction and those who are on palliative care, are the main target patients for pixantrone, which is approved by the EMA and mentioned in the ESMO guidelines (in heavily treated patients)2 and by NICE (in patients receiving third- or fourth-line treatment who have previously received rituximab),3 primarily on the basis of the results from the PIX301 study.
PIX301 was a multicentre, randomised, active-controlled study evaluating the efficacy and safety of pixantrone as a single-agent therapy in the management of patients with aggressive RR-NHL who had received at least two prior therapies (one of which had to have contained an anthracycline); patients had to have had a 3-month response to that anthracycline to be eligible.23 Patients were randomly assigned to treatment with pixantrone dimaleate or to a comparator (vinorelbine, oxaliplatin, ifosfamide, etoposide, mitoxantrone, or gemcitabine) given at standard single-agent therapeutic doses and schedules. The results of the study showed a significant improvement in responses (complete response/unconfirmed complete response [CR/CRu] and ORR) and a trend to longer duration of response with pixantrone versus active comparator agents. Pixantrone was also effective in patients who had received a significant lifetime dose of prior anthracyclines. Importantly, from a clinical point of view, it was observed that most patients who were going to respond had experienced some response by two cycles, thereby avoiding treatment and toxicity for patients who would not derive benefit from the treatment. A post hoc analysis of the PIX301 study showed that the efficacy of pixantrone (improvement in ORR and progression-free survival) versus a comparator was independent of previous rituximab therapy.24 In terms of toxicity, pixantrone has a predictable and manageable safety profile, with the main toxicity being neutropenia. Patients on pixantrone stayed in the study longer than those in the comparator arm, with no significant cardiac toxicity (a common toxicity with anthracyclines). This was thought to be due to the distinct mechanism of action of pixantrone compared with other anthracyclines; rather than acting through topoisomerase II to induce apoptosis, pixantrone appears to induce cell death through accumulation of aberrant cell divisions.25
In summary, pixantrone has been demonstrated to have efficacy as a single agent for the third- or fourth-line treatment of multiple RR-aggressive B cell NHL, with a predictable safety profile. The benefit of pixantrone has not been formally established for fifth-line or greater chemotherapy in patients who are refractory to last therapy. The structure and mechanism of action of pixantrone is distinct from anthracyclines, with a promising cardiac toxicity profile. It is the first and only EMA-approved therapy in this setting, and studies of combination therapy are ongoing.
Implementing a New Therapeutic Option
Professor Pier Luigi Zinzani
Although aggressive B cell NHL has a cure rate of approximately 50–60%, relapse within the first 2 years following initial therapy is common. There is no approved treatment or standard of care for patients who fail first- and second-line treatment. Market research among clinicians in the European Union (EU) demonstrated that nine or more different regimens may be used in the third- and fourth-line setting. The life expectancy of the multiple relapsed population is poor; as such, there is a significant unmet medical need in multiple RR patients.26 Indeed, according to the algorithm for aggressive NHL in the EU (Figure 1), there is a large population of patients who would be suitable for treatment with pixantrone; in particular, heavily pretreated patients from the third-line or patients who are ineligible for autologous transplantation.
A large number of targeted agents are being evaluated for the treatment of DLBCL (Table 1),23,27-34 including phosphoinositide 3-kinase (PI3K) and Bruton’s tyrosine kinase (BTK) inhibitors such as idelalisib, copanlisib, and ibrutinib. There are some interesting preliminary data on the potential role of ibrutinib as a single agent, particularly in ABC DLBCL, and the final data of the Phase III randomised PHOENIX trial (NCT01855750) are awaited. On the other hand, the data for the new humanised anti-CD20 monoclonal antibodies (obinutuzumab and ofatumumab) and for the antibody-drug conjugates (polatuzumab or brentuximab vedotin) are not very encouraging. Finally, preliminary data from the Phase I/II trials on checkpoint inhibitors like nivolumab or pembrolizumab demonstrate an ORR of <20% to 25% in RR-DLBCL.
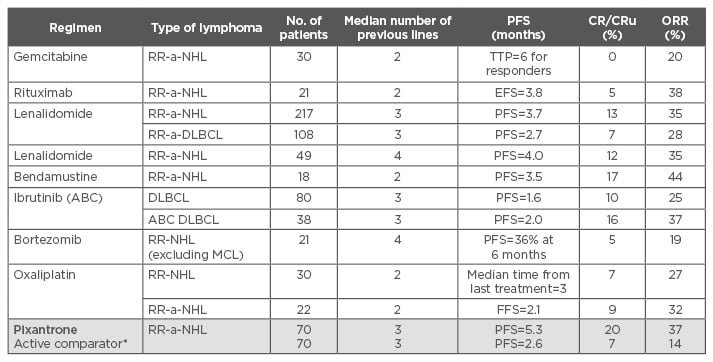
Table 1: Single-agent therapy in relapsed/refractory-non-Hodgkin lymphoma or relapsed/refractory-diffuse large B cell lymphoma.23,27-34
*Oxaliplatin, ifosfamide, vinorelbine, etoposide, mitoxantrone, gemcitabine.
ABC: activated B cell-like; a-NHL: aggressive non-Hodgkin lymphoma; a-DLBCL: aggressive diffuse large
B cell lymphoma; CR: complete response; CRu: unconfirmed complete response; EFS: event-free survival; FFS: failure-free survival; MCL: mantle cell lymphoma; ORR: overall response rate; PFS: progression-free survival; RR: relapsed/refractory; TTP: time to progression.
In terms of single-agent therapy in RR-NHL or RR-DLBCL, pixantrone has been shown to be very active with a comparable or better CR/CRu rate than other agents (Table 1), with a manageable toxicity profile and the potential for use in patients who are close to reaching their threshold for maximal anthracycline cumulative dose. Comparisons of studies showed that the CR observed for pixantrone as a single agent or in combination regimens (e.g. R-CPOP [rituximab with cyclophosphamide, pixantrone, vincristine, and prednisone] and PSHAP [pixantrone, methylprednisolone, cisplatin, and cytosine arabinoside]) were encouraging compared with other immune-polychemotherapy regimens (Table 2).18,24,35-37 In particular, in the trial evaluating the PSHAP regimen, 6 out of 11 responding patients were able to proceed to ASCT.36
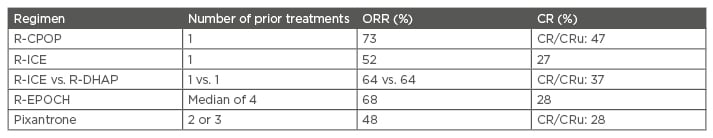
Table 2: Response rates of relapsed or refractory lymphoma to salvage regimens.18,24,35-37
CR: complete response; CRu: unconfirmed complete response; ORR: overall response rate; R-CPOP: rituximab, cyclophosphamide, pixantrone, vincristine, prednisone; R-DHAP: rituximab, cisplatin, cytarabine, dexamethasone; R-EPOCH: rituximab, etoposide, prednisone, vincristine, cyclophosphamide, hydroxydaunorubicin; R-ICE: rituximab, ifosfamide, carboplatin, etoposide.
Thus, pixantrone monotherapy can be a treatment option in RR-aggressive B cell NHL, and the combination with other chemotherapy drugs appears to be safe and effective. A pixantrone-based regimen may represent a new bridge to transplant in selected elderly patients.
A multicentre UK-wide retrospective study evaluating the efficacy of pixantrone in RR-DLBCL in clinical practice reported a lower response rate than PIX301 (18% versus 24% CR/CRu, respectively), but these real-world patients had a much higher proportion of primary refractory tumours compared with the pivotal study (85% versus 57%, respectively, p<0.001) and fewer patients with an anthracycline response duration >24 weeks (71% versus 100%, respectively, p<0.001).38 Even in this subset of patients with poor prognosis, an ORR of 24% and CR rate of 10% was achieved with single agent pixantrone without rituximab.
In conclusion, the patients with DLBCL who would benefit most from pixantrone monotherapy within its current indication are the following:
- Patients relapsing after ASCT in second-line treatment
- Patients not eligible for transplantation and relapsing after second-line treatment
- Patients eligible for a bridging strategy to allogeneic SCT
Pixantrone in Daily Practice
Professor Raul Cordoba
Prof Cordoba presented two case studies of patients with B cell NHL (who had received two prior treatments) treated with pixantrone monotherapy. The case studies illustrated that complete remissions can be achieved with pixantrone in heavily pretreated adult patients with multiple RR-aggressive B cell NHL. As myelosuppression is common, blood counts should be monitored and use of recombinant haematopoietic growth factors may be considered.
Panel Discussion
Q: How do you determine the role of pixantrone in comparison with the cell-cycle checkpoint inhibitor immunotherapies such as nivolumab and pembrolizumab?
Dr Pettengell replied that as only data from early phase studies are available, there is still little evidence for how to use and combine these drugs. It is evident that, even in the era of checkpoint inhibitors and small molecules, there will still be a need for chemotherapy to reduce tumour bulk as well as to maintain remission. In vitro studies of pixantrone with ibrutinib and idelalisib have demonstrated synergy as opposed to simple additive effects, and so combinations may be feasible and safe, though currently off-label. In vitro studies with checkpoint inhibitors show enhanced activity. Ongoing trials, such as an ‘umbrella’ trial in Germany, are investigating the safety and efficacy of multiple drug combinations.
Q: Where do you see pixantrone fitting into your clinical practice, particularly in the frail elderly population?
Prof Cordoba replied that in his institution, a geriatrician in the lymphoma unit generally performs a comprehensive assessment on patients >70 years old and classifies them as a robust, frail, or palliative patient. A strategy will be put in place to achieve a response and to prolong survival in robust and frail patients. These assessments may also be necessary to identify patients that will benefit most from pixantrone.
Dr Lugtenburg replied that the available data show that pixantrone could be used in this very difficult patient population with advanced disease, who have relapsed after second- or third-line therapies, have comorbidities, and are unable to receive ASCT or allogeneic SCT.
Q: How important is it to know cell-of-origin when using pixantrone in relapse?
Prof Zinzani replied that there were no data so far to determine if pixantrone is more active in ABC or GCB DLBCL subtypes, or in both. There are several case reports concerning the activity of pixantrone as a single agent without rituximab in ABC DLBCL. He stated that pixantrone should be considered as a new chemotherapy agent due to its unique mechanism of action and should be used because of its specific clinical activity rather than just because it is less toxic than anthracyclines.
Q: If you had to choose between bendamustine-rituximab and pixantrone-rituximab, what would you choose?
Prof Zinzani replied that according to published data, the ORR for bendamustine-rituxumab in RR-DLBCL ranged from 30–50%, and at least 20% obtain a CR, with median duration of response <4 months. However, he preferred to use pixantrone in these selected patients, as the results (in terms of median duration of response) were better with pixantrone monotherapy (without rituximab). The final data concerning the role of pixantrone plus rituximab in an ongoing Phase III study are eagerly awaited due to potentially more beneficial clinical responses.39
Dr Pettengell replied that in terms of evidence-based medicine, pixantrone was the only drug with a licence in this setting and has been evaluated in a randomised Phase III trial. She agreed with Prof Zinzani that the evidence was better for pixantrone compared with bendamustine-rituxumab.
Prof D’Amore took this opportunity to briefly describe an ongoing open-label Phase I/II trial that is testing a new combination regimen using pixantrone, etoposide, bendamustine, and rituximab (in CD20-positive tumours only): P[R]EBEN. This programme has been set up on the basis of encouraging preliminary clinical experience with the pixantrone-containing regimen,40 and will assess the safety and efficacy of this combination in patients with relapsed aggressive NHL (EudraCT number: 2015-0007).
Q: Can pixantrone be used in primary refractory patients, or should it be used only in selected patients?
Dr Pettengell said that patients had to have had a 3-month response to an anthracycline to see the results obtained in the PIX301 study. Therefore, patients who are anthracycline-refractory and have progressed through every line of therapy, or patients not fit for chemotherapy, may not do well with pixantrone (or with any novel agents being evaluated in this setting). Due to the predictable toxicity profile, it can be considered for fit elderly patients. Nonetheless, it may be worth trying pixantrone as any response will be detectable by two cycles of treatment; in the absence of an early signal, the drug can be discontinued thereby avoiding unnecessary toxicity without benefit.
Q: Is there any subset analysis information from the PIX301 study to indicate whether early responders are the ones doing best?
Dr Pettengell replied no, as the patient subsets are too small to give any meaningful answer.
Q: Are there any data on the role of pixantrone on the response rates in mantle cell lymphoma?
Dr Lugtenburg and Prof Zinzani were not aware of data regarding the role of pixantrone in mantle cell lymphoma.
Dr Pettengell mentioned some anecdotal single cases. Anthracyclines are active in mantle cell lymphoma, but only have a role in second-line given the availability of ibrutinib and idelalisib. However, pixantrone or gemcitabine may be added to the regimen in patients who progress on those drugs to prevent the rapid progression that occurs when BTK or PI3K inhibitors are stopped.
Conclusion
Despite the increased knowledge of disease biology and the development of new drugs within the last 10 years, there have not been any significant improvements in outcome for patients with RR-DLBCL. Pixantrone has emerged as an effective treatment, even as single-agent therapy, and has significant promise in combination studies. It is effective in treating patients who are older or with comorbidities, and also as a bridging therapy to consolidate autograft and allograft transplants (and perhaps radiotherapy), with the possibility of maintenance treatment following with other drugs.








