Meeting Summary
Cancer care has undergone rapid changes in recent years, providing dramatically improved outcomes for many patients. However, these changes have resulted in substantial increases in the costs of care in some situations. This symposium brought together a multidisciplinary faculty of experts in oncology, patient advocacy, hospital pharmacy, and health economics to discuss current issues of affordability and improving patient access to oncology medicines. The aim of the symposium was to understand what value truly means with regard to cancer care, consider what could happen when the cost of cancer care becomes unsustainable, and propose solutions to ensure optimal cancer care now and in the future.
In healthcare, it is no longer sufficient to demonstrate the effectiveness and cost-effectiveness of treatment. Now, issues of value, evidence-based decision-making, and quality must also be considered. The emerging paradigm of population and personalised healthcare was discussed by Prof Sir Gray, who highlighted the basic concepts of value-based healthcare and the need for improvement through collaborative systems and networks. The right of all patients to have equitable access to the best treatments and care was discussed by Geoffrey Henning. Among the potential solutions available, patient knowledge and empowerment will be of utmost importance, and co-ordinated campaigns by, and on behalf of, patients have the potential to change legislation for the benefit of patients.
Prof Aapro considered how cost savings from the increased use of biosimilar medicines might be re-invested to improve access to other medications, and Jatinder Harchowal provided examples of how pharmacists can improve system-wide efficiencies, thus establishing and embedding value at a fundamental level. Finally, Prof Jönsson provided an overview of the burden, cost, and cost-effectiveness of cancer management, highlighting the growing importance of appropriate economic evaluations in the new paradigm of value-based healthcare.
The session demonstrated that through the actions of patients and healthcare professionals as equal partners, a shift towards value-based healthcare and a culture of stewardship can be achieved. Importantly, these changes are necessary to safeguard the future sustainability of cancer care.
Introduction
Currently, affordable cancer care is at a crossroads. Unprecedented advances in cancer detection and treatment, together with growing and ageing populations, mean that healthcare budgets can no longer keep pace with escalating costs. To ensure that the costs of cancer care do not outweigh the benefits, existing resources must be optimally deployed to deliver value-based care. This requires collaboration across disciplines, balancing patient needs against those of the hospital and wider healthcare system, and the elimination of tests and interventions that are of little or no value.
The Urgent Need for Value-Based Cancer Care
Professor Sir Muir Gray
Society currently faces three major healthcare problems: unwarranted variation, the underuse of high-value interventions, and the overuse of lower or zero-value interventions.
Wide variation is present in many aspects of healthcare. If that variation is harmful for patients, their families and carers, and the health services that support them, it is unwarranted and unacceptable. Due to the complexities of healthcare systems, unwarranted variation in healthcare cannot be explained by the type or severity of illness or by patient preferences alone.1 Unwarranted variation must be addressed if high-value health services are to be provided within a set budget. Investigations of the causes of unwarranted variation have revealed two main problems. The first is the underuse of high-value interventions, especially among poorer populations, which can result in preventable disability, death, and inequity. The second is the overuse of lower or zero-value interventions, which can result in a waste of resources and, ultimately, patient harm (Figure 1).
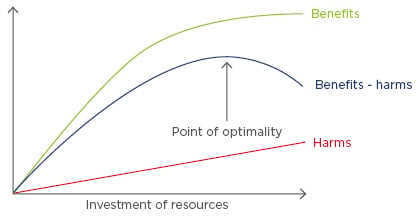
Figure 1: The pattern of benefits and harms following increasing investment of resources.
The benefits of high-quality healthcare increase with the investment of resources until optimal benefits are achieved, after which benefits decline.2
It is estimated that the demand for cancer care will increase by about 20% over the next decade. To reduce this need and improve efficiency, measures should be put in place to prevent disease, disability, dementia, and frailty; improve outcomes by providing only effective evidence-based interventions; and increase productivity by reducing cost. However, the most important focus should be on increasing value. Three different types of value should be considered:
- Allocative value: have we allocated resources to different groups equitably and in a way that maximises value for the whole population? This is determined by how well assets are distributed to different subgroups that can be defined by clinical condition, such as cancer, or by a characteristic, such as having multiple morbidity and frailty. Decisions need to be more explicit not only regarding the amount of resource allocated to patients with cancer but also within the cancer budget. One way to allocate resources is by type of cancer, reviewing whether the allocation is optimal between, for example, services for patients with breast cancer versus patients with gastrointestinal cancer. The other allocative decision is by treatment modality: is there optimal allocation in the budgets for chemotherapy, radiotherapy, and surgery? In an era of growth, these may be implicit, but in an era in which need and demand outstrip resources, the value from different patterns of investment needs to be calculated and debated.
- Technical or utilisation value: determined by how well resources are used for all the people affected within a population. Improving the quality and safety of healthcare increases the value derived from resources allocated to a particular service.
- Personalised value: basing decisions on the best current evidence, a careful assessment of an individual’s clinical condition, and an individual’s values. These are the values they place on good and bad outcomes, because even the highest quality healthcare can do harm; therefore, patients need to be provided with full information about the risks and benefits of the intervention being offered. For example, there would be little value for patients to gain a few weeks of life at the expense of extreme nausea.
The introduction of ‘triple-value healthcare’ has the potential to cultivate a culture of stewardship, whereby clinicians can realise and hold themselves accountable for value-based healthcare principles. Such principles include shifting resources from budgets where there is evidence of overuse or lower value to budgets for populations in which there is evidence of underuse and inequity, ensuring that specialist services are reserved for those who would benefit most, and using the cost-savings generated from reduced spending on interventions that are of limited value to fund innovation and increase the use of high-value interventions (Figure 2).
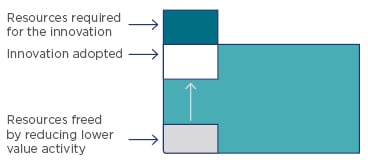
Figure 2: Population-based systems that implement high-value innovations funded by reduced spending on lower-value interventions in the same programme budget.
While there is a need to continue evidence-based decision-making, prevention, and quality improvement, these decisions should also involve individuals to ensure that they are right for the particular values and condition experienced by each patient. Maximising value for populations and the individuals within them will require time and multi-stakeholder engagement to ensure that all perspectives are accounted for.
Putting the Patient First: Showing the Unacceptable Levels of Inequality of Access Across Europe
Geoffrey Henning
Universal access to health services is a commitment made by all European Union (EU) member states, yet this principle has not prevented substantial inequalities in access to healthcare across Europe. In fact, health and access to healthcare in Europe are strongly determined by socioeconomic status. According to the Organisation for Economic Cooperation and Development (OECD) statistics, expenditure on drugs and other perishable goods for 2014 ranged from a high of $618 per capita in Germany to as low as $114 per capita in Poland.3 Indeed, patients with metastatic breast cancer in Eastern Europe have far less access to medicines than similar patients in Western Europe. Between 2012 and 2014, while around 11,800 patients in Poland were diagnosed with HER2+ breast cancer, only 5,100 women had access to the appropriate medicine.4
Biosimilars represent a real opportunity to increase patient access to safe and effective medicines, but patient concerns will need to be addressed for the nature and benefit of these medicines to be realised. Patients have a right to know which medicines they are prescribed and need to be informed when their medicine has been switched or substituted. Appropriate discussions are needed and full information about biosimilars should be made available for all patients so that they understand the issues surrounding them, along with the opportunities they offer. The challenge will be pricing and whether biosimilars will be available for all patients or remain out of reach for some.
Copayments are an option in many European countries; however, there is a danger that these put increased financial pressure on families, potentially leading to worse outcomes. A distinction regarding copayment could potentially be made according to value, with therapies considered of high value automatically funded and those of lower value considered for copayment.
In terms of changing the current situation, patient empowerment is crucial. Together, clinicians and patients represent a formidable force for change in health systems, and their united voice should be heard where inequalities exist. Through knowledge and collaboration, co-ordinated campaigns have the power to change legislation, helping to ensure access to the best treatment and care for all EU citizens.
As Oncologists, What Can we do to Improve Value?
Professor Matti Aapro
There is no evidence that spending more on cancer consistently improves outcomes. In 2010, a study found that Sweden and Finland had almost identical 5-year colorectal cancer survival rates (approximately 59%). However, according to the calculations presented in the paper, the total expenditure per colorectal cancer case was approximately €10,000 in Sweden versus €172,000 in Finland, equating to a 17-fold difference in expenditure per case with no evidence of benefit.5
According to the European Society for Medical Oncology (ESMO), the value of any new therapeutic strategy or treatment is determined by the magnitude of its clinical benefit balanced against its cost.6 However, whereas costs vary from country to country, the magnitude of clinical benefit, as derived from well-designed clinical trials, is a relative constant. The aim of the ESMO Magnitude of Clinical Benefits scale, therefore, is to assess the clinical benefit of different cancer medications. This will allow stakeholders to distinguish between treatments that bring substantial improvements in the duration of survival and/or quality of life of cancer patients and treatments with benefits that are more modest, limited, or even marginal. In short, it aims to identify which interventions are of high value: knowledge that should help to minimise the use of low-value treatments. In doing so, therapies that provide little, or no, patient benefit can be avoided and the overuse of treatments and/or misuse of tests reduced.7,8
One approach to increasing value-based healthcare is through the use of more affordable medications. Biosimilar medicines have equivalent efficacy and safety to already approved reference medicines, but may be more affordable. In the UK, the availability of biosimilar filgrastim resulted in a 30% increase in overall filgrastim use from 2008 to 2010, contributing to a shift in treatment practice from secondary prophylaxis towards increased primary prophylaxis.9 Switching to biosimilar filgrastim in a community setting resulted in an increase from 36% to 52% in the use of filgrastim as primary prophylaxis.10 Furthermore, between 2006 and 2013, the treatment volume of filgrastim per capita increased by an average of 44% across the EU.11 These data suggest that the use of more affordable medicines may give more patients access to important treatments.
Cost-savings generated from the use of biosimilar medicines, and other more affordable treatments such as small molecule generics, can also be reinvested to improve patient access to other, more expensive, treatments. For example, it has been estimated that a 100% switch to a biosimilar epoetin for oncology indications in seven European countries (France, Italy, Spain, Germany, Romania, UK, and the Netherlands) in 2010 would have resulted in a total of $188 million being saved annually.12 This may enable increased access to potentially life-saving drugs. A saving of $188 million would, for example, have supported rituximab treatment for approximately 9,000 extra patients.12
The Pharmacist as an Agent of System-Wide Change
Jatinder Harchowal
The current pipeline for oncology medicines will result in a plethora of new agents coming to market, with an associated increase in overall costs. Conversations around value-based pricing based on outcomes have, therefore, become commonplace, with questions arising on how value should be defined. In such situations, collaborative leadership is required, and all aspects of the patient pathway considered.
At the Royal Marsden Hospital, London, UK, there are approximately 50 pharmacists, almost 20 of whom have an advanced practice role, which enables them to review and prescribe chemotherapy agents and review supportive medications. Such pharmacists add value to patient care by optimising the use of medicines as part of a multidisciplinary team. They play a key role in reducing medication errors, inappropriate polypharmacy, preventable medication-related harm, and poor adherence. In many cases, non-adherence is the result of adverse events; therefore, monitoring safety is an especially important component of the pharmacist’s role.
Pharmacists are also increasingly involved in the implementation of system-wide efficiencies, with the aim of introducing value-based care. An NHS England initiative on the standardisation of chemotherapy, for example, introduced national dosing bands for the 50 most commonly used cancer drugs. Compared with the previous system, where dose calculations varied slightly according to each cancer centre’s protocol, the new standardised approach provides significant efficiencies. These include doses being stocked as batches, the ability to offer off-the-shelf treatment to new patients or patients in urgent need, less wastage, and fewer delays to the patient receiving treatment.
In 2015, the Cancer Vanguard programme was launched to test and fast-track innovative models of cancer care, as recommended by the NHS National Cancer Strategy. Pharmacy teams from the main sites of the Cancer Vanguard programme worked collectively to produce a centralised repository of information to help support the introduction of biosimilar rituximab across NHS England. This included a co-ordinated educational approach, developing an interactive PDF to educate staff, an information sheet for patients, and policy guidance for the entire country. Most patients have now been switched from reference rituximab to biosimilar rituximab. In 2016, £186 million was spent in England on rituximab across all its indications. It has been estimated that NHS England will save at least £65 million in oncology alone by switching to the biosimilar medicine over the next year. Such savings can be used to fund the purchase of additional medicines, thus increasing patient access, and to employ additional staff that can help manage the overall patient journey.
Leveraging Heath Economic Data
Professor Bengt Jönsson
To assess the sustainability of cancer care, data are needed on three key economic variables: the burden of cancer, the cost of cancer, and the cost-effectiveness of cancer management. Between 1995 and 2014, the incidence of cancer increased by 30%, largely due to population growth and ageing13 and as such, the burden of cancer has also increased. One measure of the overall burden of a disease is the disability-adjusted life year (DALY), which equals 1 year of healthy life lost. In 2012, 19% of DALY in Europe were due to cancer, second only to cardiovascular disease (21%).14 In many countries, cancer has recently overtaken cardiovascular disease as the leading cause of DALY, suggesting that in the near future, cancer is likely to be the main contributor to disease burden across Europe. The proportion of spending on cancer, however, does not reflect its disease burden. In the EU, health expenditure on cancer increased from €35.7 billion in 1995 to €83.2 billion in 2014, with spending on cancer drugs increasing from €7.6 billion in 2005 to €19.1 billion in 2014.13 Despite these increased costs, the share of total health expenditure devoted to cancer (around 6%) has changed little over the last 30 years in both Europe and the USA. This may be explained by other expenditures, such as inpatient care, having decreased.
Wide variations in the cost of cancer care exist across Europe, even between countries of similar economic strength. For chronic myeloid leukaemia, data show that Spain spends €20,000 per case, Germany €16,000, France €14,500, Italy €13,000, and the UK €10,000.14 These data suggest some countries are overspending and others underspending. Furthermore, for tyrosine kinase inhibitors, which have dramatically changed outcomes in chronic myeloid leukaemia, data show that the defined daily dose given to patients varies according to a country’s gross domestic product (GDP). The defined daily dose for tyrosine kinase inhibitors was 375 mg for countries in the lower GDP tier, 500 mg for those in the mid GDP tier, and 590 mg for those in the upper GDP tier.14 Therefore, when prices decrease, it will be countries with the lowest incomes that will benefit most.
With limited healthcare resources, greater emphasis is needed on achieving value for money. To understand the value of treatments, data on health economic outcomes must be captured in clinical trials and must also be generated post-launch. To help support decisions on the value of medicines, information and evidence from health technology assessments must be fed into decisions around priorities and guidelines, and the impact of these measures continuously reviewed and updated (Figure 3).
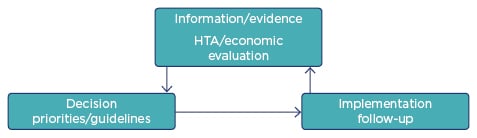
Figure 3: The circle of follow-up to ensure value for money.
An illustration that the right decision is necessary but not wholly sufficient.
HTA: health technology assessment.
The need for long-term follow-up of reimbursement decisions is supported by the UK Cancer Drug Fund (CDF), which, between 2010 and 2016, cost £1.27 billion. Of the 47 CDF approved indications, a statistically significant benefit in overall survival was only seen in 18 (38%), with a median survival of 3.1 months (1.4–15.7 months).15 Furthermore, when assessed according to clinical benefit scales, only 23 (48%) and 9 (18%) indications met American Society of Clinical Oncology (ASCO) and ESMO criteria for clinical benefit, respectively. The National Institute for Health and Care Excellence (NICE) had previously rejected 26 (55%) of the CDF approved indications, because they were not considered cost-effective; however, no additional data on patient outcomes were collected, which highlights the need for real-world evidence on outcomes and resource use.
Conclusion
The symposium provided a great opportunity to demonstrate that the future of sustainability in cancer care requires ongoing effort from multiple stakeholders throughout the healthcare system, from patient to health economist. With collaborative effort, increasing implementation of value-based healthcare can, and must, be achieved.








