Abstract
Giant cell arteritis (GCA) is the most common systemic vasculitis. In the past two decades there have been significant advancements in our understanding of the pathophysiological mechanisms underlying the disease, and consequently the management of GCA is evolving. GCA is a medical emergency because when left untreated it can lead to devastating complications including irreversible visual loss. Thus, prompt diagnosis is imperative to ensure appropriate treatment and prevent ischaemic events. However, uncertainty remains over diagnostic pathways, including appropriate modalities and standardisation of findings. Temporal artery biopsy has been considered the gold standard diagnostic test but has significant limitations in terms of false negative results. In recent times, several new diagnostic modalities have been proposed in GCA including temporal artery ultrasound, CT angiography, magnetic resonance angiography, and PET. In this paper, the authors review the advantages and limitations of current diagnostic modalities in GCA.
![]()
Corrigendum: Giant Cell Arteritis: Navigating Beyond the Headache
Authors: Patricia Harkins, Richard Conway
Original citation: EMJ Radiol. 2021; DOI/10.33590/emjradiol/20-00146
Date correction published: 18.01.21
The article by Harkins and Conway et al. in an article of EMJ Radiology was originally published on 18.01.21. Since then a corrigendum has been made. References 70 had not been cited in the text. This has now been updated in all versions of the article.
The EMJ apologises for the error and any inconvenience caused
![]()
INTRODUCTION
Giant cell arteritis (GCA) is an idiopathic granulomatous vasculitis involving medium and large calibre arteries.1 It is the most common systemic vasculitis, with a female preponderance, and occurs almost exclusively in those >50 years of age.2,3 It occurs mainly in Caucasian populations, with the highest incidence in those of Northern European descent.4 With the worlds ageing population, the number of patients diagnosed with GCA by the year 2050 is predicted to be in excess of 3 million, of whom approximately 500,000 will be visually impaired.5 The systemic nature of GCA was noted as far back as 1938 in a paper by Jennings;6 however, the traditional description of GCA has focussed on cranial symptoms resulting in its misclassification as a ‘headache disease.’7 In the past two decades the importance of the overlapping GCA phenotype, including cranial GCA and large vessel GCA (LV-GCA), has been recognised.4 Although advances in imaging modalities have raised awareness for the frequency of LV-GCA, there remains a paucity of data guiding the modality of choice in both diagnosis and disease activity assessment.
GCA remains a clinical diagnosis, supported by laboratory and imaging investigations. The most recent British Society for Rheumatology (BSR) guidelines highlighted the importance of clinical acumen in stratifying further investigation, emphasising the importance of pretest probability in guiding the diagnostic pathway.8 A new-onset headache is the most common systemic symptom,9 with jaw claudication being the most specific presenting symptom.9-11
Early and accurate diagnosis of GCA is imperative. Failure to accurately diagnose and expeditiously treat GCA may lead to devastating complications, including permanent visual loss, which occurs in 15–20% of patients.12 Misdiagnosis, on the other hand, can lead to inappropriate glucocorticoid use and the toxicities associated with this.13
TEMPORAL ARTERY BIOPSY
Temporal artery biopsy (TAB) for histological analysis has traditionally been the gold standard for the diagnosis of GCA.14,15 Typical histological features in GCA include granulomatous inflammation in all layers of the muscle wall, with a predominantly T-lymphocyte and macrophage-rich population localised between the media and adventitia.7 Other, less frequently identified histological patterns include inflammation limited to the vasa vasorum, the adventitia, or inflammation targeting small periadventitial vessels with no muscular coat, which therefore spare the temporal artery (TA) itself.16
When present, multinucleated giant cells are usually localised in close proximity to a fragmented internal elastic lamina; the presence of neutrophils, eosinophils, and plasma cells are rare.7 Although highly specific, it is not a perfect test as it is relatively invasive and has poor sensitivity, with false negatives reported in up to 61% of patients compared to a clinical diagnosis of GCA.17 This can be partly attributed to the segmental nature of the vasculitic involvement by GCA with so called ‘skip lesions’.18 To account for this, the BSR recommends that all TAB specimens be at least 1 cm in length postfixation.19 There is at least a 10% shrinkage rate of TA segment length reported after formalin fixation20 and therefore, optimal TAB length should be at least 1.5 cm.21
To date, ultrasound-guided biopsy has failed to demonstrate improved sensitivity or yield of TAB,22 and biopsy of the contralateral TA has been reported to only provide a modest improvement in diagnostic yield and so is not routinely recommended.23 Furthermore, the administration of glucocorticoids may affect the sensitivity of TAB results. In the TABUL study, sensitivity fell from 48% to 33% when TAB was performed within 3 days of glucocorticoid commencement versus within 7 days of glucocorticoid commencement, respectively.24 However, Maleszewski et al.25 demonstrated histologic activity at 420 days post glucocorticoid initiation.
With increasing knowledge of the diverse phenotypic nature of GCA, and the understanding that the temporal arteries can be spared in up to 40% of cases of extracranial LV-GCA, coupled with the issues outlined above, there has been a transition to other diagnostic modalities to optimise diagnosis in GCA. The most recent European League Against Rheumatism (EULAR) consensus proposes the use of colour Doppler ultrasound (CDUS) in the first-line in patients with suspected large vessel vasculitis, in particular cranial GCA (cGCA), provided it is promptly available and the test is performed and interpreted by a trained, experienced specialist using the appropriate machine settings and protocols.25 The BSR guidelines also recommend CDUS in the first instance, with the necessity of TAB being determined by clinical pretest probability.8
COLOUR DOPPLER ULTRASOUND
A growing body of literature over the past two decades have provided robust evidence for the routine use of CDUS in the diagnosis of GCA. It allows assessment of the whole length of the superficial temporal arteries, potentially overcoming the issue of skip lesions affecting histological results.10 Furthermore, unlike TAB, which is limited to one anatomical region, CDUS can be used to evaluate accessible cranial and extracranial vessels, increasing the diagnostic yield for this systemic pathology.
When examining the TA in a suspected case of GCA, it is recommended that the complete length of the common superficial TA, with its frontal and parietal branches in transverse and longitudinal planes, be examined bilaterally.26 In addition to this, studies have demonstrated that the axillary arteries (AX) are the most frequently involved extracranial vessels accessible by CDS, and that when affected the inflammation is often present bilaterally. Therefore, examining the AX and TA bilaterally can increase the diagnostic yield in GCA and is recommended in all cases.27 Further arteries may be examined if the diagnosis remains unclear or if the clinical assessment of patient history and examination points to the involvement of other arterial territories, such as lower limb intermittent claudication and involvement of the femoral or popliteal arteries.
While promising efforts have been made recently to devise quantitative CDUS criteria for the diagnosis of GCA, they have thus far failed to be incorporated into guidelines or to gain momentum in clinical practice.28,29 Instead, qualitative assessment measures for interpreting CDUS findings have continued to be used. A normal intima–media complex on TA ultrasound examination reveals a homogenous, hypoechoic, or anechoic structure delineated by two parallel hyperechoic margins.30 Upon CDUS examination of an affected TA in GCA, the hallmark ‘halo sign’ is diagnostic, defined as a homogenous, mostly concentric, hypoechoic wall thickening, well delineated toward the luminal side, visible in both longitudinal and transverse planes (Figure 1).13
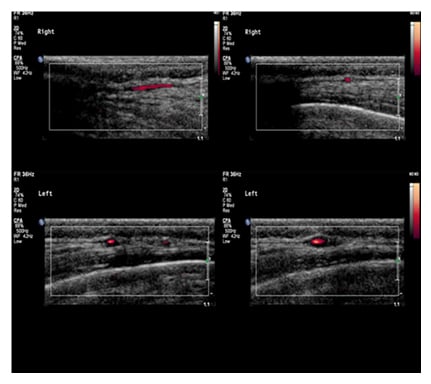
Figure 1: Colour Doppler ultrasound examination of a normal and an affected temporal artery in giant cell arteritis.
A unilateral halo sign has a sensitivity of 68% and a specificity of 91%, with this increasing to a specificity of 100% with the presence of bilateral halos.31 The thickened wall of a vasculitic artery remains persistently visible upon compression of the lumen with the ultrasound probe, giving rise to the so called ‘compression sign’. This demonstrates excellent interobserver agreement, with a sensitivity of 75–79% and a specificity of 100% for the diagnosis of GCA.32,33 The external validity of these more specialised tests requires further assessment. The presence of the halo and compression signs have been regarded by the OMERACT Large Vessel Vasculitis Ultrasound Working Group as the most important CDUS findings suggestive of vasculitis, and the presence of the halo sign is also specified as a minimum requirement for the diagnosis of GCA.15 The presence of stenosis and occlusion were previously thought to aid the diagnosis of GCA; however, with advancements in the resolution of ultrasound equipment, these findings have been demonstrated to be neither sensitive nor specific.12,34
Compared to TAB, CDUS is a safe, well tolerated, noninvasive bedside procedure, that affords the advantage of almost instant results. Furthermore, versus all imaging modalities employed in the diagnosis of vasculitis, CDUS has the highest resolution (100 μm).35 Images and videos taken during CDUS examination can be stored and readers of these have been shown to achieve the same reliability as that of a pathologist evaluating a biopsy specimen.9 However it is very much operator dependent and requires skilled sonographers with expertise in the area of temporal artery CDUS.36Similar to other diagnostic modalities, the sensitivity of CDUS in the diagnosis of GCA diminishes upon initiation of glucocorticoids.37 The TABUL study demonstrated a significantly smaller halo size in those who had received >4 days of glucocorticoid therapy versus those who had received up to 4 days of glucocorticoid therapy.9 It is thus imperative that CDUS be performed as close to the initiation of glucocorticoids as possible. This has led to the development of ‘fast track clinics’ in centres with expertise in the management of GCA, where patients are ideally seen within 24 hours of initial suspected GCA presentation.22,38,39 They undergo a clinical assessment, including structured history and clinical examination, in addition to an ultrasound examination of the TA and AX by an experienced rheumatologist.
One of the main advantages of this clinic is its ability, in the majority of cases, to either exclude GCA, or provide a rapid and clear diagnosis of GCA and thus initiate prompt and appropriate specialised care. This reduces the exposure to harmful complications of unnecessary glucocorticoid treatment, but also has been shown to optimise patient outcomes in those with GCA. Two retrospective studies have demonstrated how, with the inauguration of fast track clinics, the rate of permanent vision loss fell dramatically from 37% to 9%39and from 19% to 2%.40 The recent TABUL prospective multicentre cohort study assessing CDUS performance in a rigorously controlled setting reported a CDUS sensitivity of 54% and a specificity of 81% compared to physician diagnosis at 6 months.19 Although superior to prior studies, it similarly focussed on a specialist centre with experienced operators; thus, it potentially fails to capture a real-world assessment of CDUS performance. The authors highlighted this in a recent study where they assessed the CDUS performance characteristics of TA in routine clinical practice with a heterogenous patient population with variable delays between symptom onset, treatment initiation, and diagnostic imaging.40 They showed a CDUS sensitivity of TA of 52.8%, with a specificity of 71.8%. The sensitivity of testing significantly increased to 78.9% when TAB was used with CDUS of TA, with no change in specificity. Although more reflective of real-world performance, this study also used experienced operators of CDUS in the diagnosis of GCA and so arguably fails to represent the majority of centres, in which CDUS will be performed by radiologists whose skills are also required in multiple competing areas. Therefore, both these findings and those of TABUL stress that, whilst valuable in the diagnosis of GCA, CDUS should be used with caution and by those highly experienced in the assessment of GCA.
Contrast-enhanced ultrasound (CEUS) is evolving as an increasingly popular technique in vascular imaging and the potential use of carotid CEUS in the diagnosis of large vessel vasculitis (LVV) has recently been explored.41-43 A small prospective study demonstrated the use of carotid CEUS in the assessment of carotid wall neovascularisation and its ability to act as a potential surrogate marker of disease activity in those with LVV.45 Furthermore, when compared with PET imaging, carotid CEUS demonstrated a strong correlation in identifying and grading vascular inflammation in a small population with LVV.44 Although further studies with a higher power are required, this is a potentially exciting noninvasive method to detect and monitor disease activity in those with GCA.
PET SCANNING
PET is a technique employed since the late 1990s in the diagnosis and assessment of LVV. It is a functional nuclear medicine imaging technique which uses fluorine-18-fluorodeoxyglucose (F-18-FDG), a positron-emitting radionuclide, that shows increased uptake in metabolically active cells such as those in infection, malignancy, and inflammation.45 F-18-FDG does not accumulate in normal vascular structures, and so any evidence of uptake within the vasculature tree is considered abnormal, with increased F-18-FDG uptake in the vessel wall being the hallmark of vasculitis in PET. F-18-FDG uptake typically following a symmetrical pattern in GCA and bilateral arteries tends to always be involved to the same extent.46 In current practice, PET is almost exclusively combined with CT (PET-CT), working synergistically to assess the morphological structure and metabolic function of the vascular territories.47 In more recent times, PET has also been combined with MRI (PET-MRI).
The EULAR Recommendations Working Group for the use of imaging in LVV recommend the use of PET (or ultrasound, MRI, or CT) for the detection of mural inflammation in extracranial arteries to support the diagnosis of large vessel GCA.10 A major limitation of PET in the diagnosis of GCA has been the lack of spatial resolution to distinguish small branches of the external carotid artery, including the TA, from the background high physiological uptake in the brain. However, improved PET/CT technology allows time of flight (TOF) reconstruction, which improves image quality and provides a greater signal to noise ratio, allowing for arteritis detection in the smaller extracranial arteries of the head and neck, including the TA.48-50 Thus, TOF PET/CT can now be used to diagnose areas of arteritis in the whole body, including the previously beyond-resolution small branches of the external carotid artery, while also reducing radiation exposure and scan time.51
The GCA and PET scan (GAPS) study, a double-blinded, prospective, cross-sectional study utilising a TOF scanner with 1 mm CT reconstruction, aimed to assess the use of PET as a first-line diagnostic tool in GCA.40 It demonstrated that, versus TAB, the sensitivity and specificity of PET/CT was 92% and 85%, respectively, with a negative predictive value of 98%. Compared to clinical diagnosis, PET/CT had a sensitivity of 71% and a specificity of 91%. The finding of a negative predictive value of 98% demonstrated the use of this imaging technique in ruling out GCA in those considered lower risk. It is also worth noting how, whilst ruling out GCA, PET/CT has the ability over other diagnostic methods of detecting vasculitis mimics, infection, and malignancy, and has demonstrated a clinically relevant incidental finding in 20% of those enrolled in the study.40 Furthermore its use in the identification of a clinically silent LVV in those with polymyalgia rheumatica has been demonstrated in numerous studies, with PET/CT revealing LVV in up to one-third of those with perceived isolated polymyalgia rheumatica, particularly refractory and atypical cases.52-54 To date, there is no definitive internationally-accepted consensus criteria to define and interpret the presence of vascular inflammation using FDG-PET in GCA.55 To optimise the performance of this diagnostic tool, in addition to ensuring optimal comparison between centres to facilitate future multicentre trials, there is a pressing need for a standardised scoring system for vascular uptake. Visual, qualitative, semiquantitative, and quantitative methods of interpretation have all been proposed. There is no ‘gold standard’ but a time-honoured method of interpretation has been the Meller scale: a semiquantitative four-point scale grading the vascular uptake with respect to that of the liver.56 It includes the following grades: Grade 0 (no vascular uptake), Grade 1 (vascular uptake less than the liver), Grade 2 (vascular uptake similar to the liver), and Grade 3 (vascular uptake higher than the liver). A high interobserver agreement has been demonstrated in Meller Grade 3, but not in Grade 2 or 1.57 Another issue is that although increased vascular uptake is suggestive of vasculitis, it is not pathognomonic for it, with atherosclerosis also causing increased uptake. This compromises the diagnostic accuracy of FDG-PET, particularly in the case of Grade 1, and less commonly Grade 2, uptake.58 Typically, atherosclerosis has a ‘patchy’ irregular uptake pattern, with vasculitis uptake usually smooth and linear, involving long segments. Like other diagnostic methods in GCA, the shorter the interval to PET acquisition from glucocorticoid commencement, the higher the diagnostic potential.
In a recent study, 3 days of prednisolone 60 mg/day did not substantially attenuate FDG-PET results; however, 10 days of the same treatment resulted in a significant loss of vascular FDG uptake.59 Furthermore, the use of glucocorticoids has been shown to increase FDG uptake in the liver, which may result in an underestimation of vascular FDG uptake if using the liver as a comparison.60 Therefore it has been recommended that PET ideally be performed within 72 hours of glucocorticoid commencement to ensure high diagnostic potential; whilst this may be feasible in some centres, in the majority of centres the acquisition of PET scans within 3 days may not be as accessible and so may limit the use of this diagnostic tool.
CT AND MRI/MAGNETIC RESONANCE ANGIOGRAPHY IN CRANIAL GIANT CELL ARTERITIS
CT angiography (CTA) and MRI/magnetic resonance angiography (MRA) are alternative imaging modalities that identify arterial wall abnormalities reflective of vascular inflammation, such as oedema and contrast enhancement, in addition to assessing the arterial lumen for associated structural abnormalities, such as stenosis or aneurysm formation.
For those with predominantly cranial symptoms, high-resolution MRI has shown high sensitivity in the detection of cranial arteritis.61-64 MRI allows for simultaneous and continuous evaluation of the cranial artery segments.58 Using TAB as the diagnostic gold standard, MRI was demonstrated to be 94% sensitive with a specificity of 78% in the diagnosis of GCA.56 Notably, in the same study, the negative predictive value of MRI was 98% raising the possibility of a negative MRI eliminating the need for TAB.56 However, when using the diagnostic reference standard of clinical diagnosis, the sensitivity of MRI fell to 39%, with a specificity of 82%.56 This highlights the need for improved standardisation of a diagnostic reference standard in GCA. MRI of the TA has been included in the recent EULAR recommendations for imaging in GCA as a second-best alternative to CDUS to assess the cranial arteries.26
CTA has been shown to detect superficial TA abnormalities in GCA.65 The authors demonstrated, in a retrospective case control study, how CTA has a sensitivity of 71% and a specificity of 86% for the diagnosis of cGCA. The characteristic appearance of GCA in the study was of a well-defined normal vessel disappearing into an abnormal, ill-defined area of hyperenhancement, reminiscent of smoke arising from the end of a cigar (Figure 2). Like CDUS, the findings of stenosis and occlusion appeared to be nonspecific in this group.
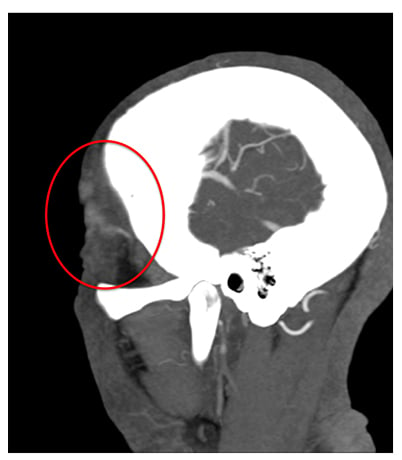
Figure 2: The characteristic appearance of giant cell arteritis of temporal artery on CT angiogram.
CT AND MRI/MAGNETIC RESONANCE ANGIOGRAPHY IN LARGE VESSEL GIANT CELL ARTERITIS
There are currently no guidelines addressing the screening need for large-artery disease and complications in GCA. In clinical practice, MRI/MRA and CTA are commonly used interchangeably to detect large vessel involvement. CTA can evaluate mural thickening, stenosis, and aneurysm formation of the aorta and branch vessels.52 It has a reported sensitivity of 73% and a specificity of 78% for the diagnosis of LV-GCA.66Although limited by the risk of contrast and radiation exposure associated with its use, CTA has the ability to detect structural lesions with a higher resolution and shorter scanning time than MRA.67
MRA may be particularly advantageous in this setting due to its ability to simultaneously assess vascular morphology, detect oedema, and contrast uptake using specific weighted sequences, without radiation exposure.47 This, combined with its good reproducibility, may suggest a role for MRI/MRA in disease monitoring in GCA, including screening for complications such as aneurysm. One study compared the role of MRA and PET in the diagnosis of LV-GCA and highlighted how each of these modalities contributes separate but complementary information regarding disease presence and extent.68 MRA was superior to PET in assessing disease extent due to detection of both arterial wall and luminal abnormalities. However, PET was shown to be more reliable than MRA in the assessment of disease activity. Of note, oedema on MRA was the strongest correlate of PET scan activity, and oedema in an increasing number of arterial territories was most predictive of an MRA being interpreted as ‘active’, providing a potential surrogate for PET scan activity in cases where PET is unavailable or contraindicated.68
Similar to the other diagnostic modalities outlined above, the performance characteristics of CTA and MRI/MRA are affected by the initiation of corticosteroids.63,69
CONCLUSION
While significant advances have been made in recent times in the diagnosis of GCA, this is still an evolving field with a large number of unmet needs. Much uncertainty still surrounds the optimal diagnostic modality and, despite diagnostic guidelines from both EULAR and BSR, the diagnostic pathway remains challenging.
Pretest probability should be used to guide the diagnostic pathway, with modalities chosen based on local availability and centre expertise. In both the diagnosis of cGCA and LV-GCA, there is an urgent need for standardisation of imaging-based measurements of vascular activity to improve patient outcomes and advance clinical research. Furthermore, a definitive biomarker for GCA diagnosis and assessment of disease activity is required, with a number of serological markers and novel PET ligands demonstrating future promise.







