Abstract
Gastrointestinal stromal tumours (GIST) account for 1–3% of all the gastrointestinal (GI) neoplasms. It is the most common mesenchymal tumour in the Gl tract. The majority of GISTs are KIT gene positive; however, it is necessary to diagnose them pre-operatively. Here, the authors report the case of a 65-year-old male who presented with pain and a lump in the umbilical region for the last 2 years. On abdominal ultrasound, there was a heterogeneously hypoechoic mass in the umbilical region, with lobulated margins and central necrotic areas. On small bowel series, the core of the mass showed faint contrast opacification. Contrast-enhanced CT of the abdomen showed a heterogeneously enhancing, lobulated exophytic lesion arising from the small bowel loops, the central core of the lesion demonstrating an air-contrast level. Under ultrasound guidance, the lesion was biopsied. Histopathological findings were suggestive of a spindle cell tumour. The sample was c-KIT positive. Hence, the diagnosis of GIST was confirmed. The patient was given imatinib after resection of the mass. Usually, a GIST of larger size has higher malignant and metastatic potential; however, this article shows a gigantic small bowel GIST with cavitation and heterogeneity in different imaging modality, and still has no metastasis on imaging or high mitotic activity, and nuclear atypia on histopathology. A radiologist should also know the imaging pattern on conventional imaging and ultrasound, apart from usual cross-sectional imaging.
Key Points
1. Although CT is the imaging modality of choice, and presence of the c-KIT gene on immunohistochemistry confirms the diagnosis, this case report emphasises the role of ultrasound and conventional imaging.2. Small bowel series and ultrasound can prove to be useful along with CT, to confirm the origin of air foci within a lesion.
3. Gastrointestinal stromal tumours (GIST) >5 cm usually show lobulated margins, central cavitation, and heterogenous enhancement on venous phase. Larger GIST tends to be malignant and metastasise to the liver, which was not seen in this case.
INTRODUCTION
Accounting for 1–3% of all gastrointestinal (GI) tumours, the most common mesenchymal tumour of the GI tract, which originates from the interstitial cells of Cajal in the myenteric plexus of the muscularis layer, is GI stromal tumour (GIST).1,2 Apart from CT, ultrasonogram, radiographs, and barium study, there are other multimodality imaging options, with which a radiologist should be well-versed. Here, the authors report a case of a 65-year-old male patient with a large small bowel GIST, highlighting the role of imaging in GIST and its management.
CASE PRESENTATION
A 65-year-old male presented with pain and a lump in the umbilical region for the last 2 years. He also had anorexia and weight loss for 2 months. He did not have fever, jaundice, or history of any previous surgeries. Clinical examination of the abdomen revealed a large abdominal lump in the umbilical region, spanning both the lumbar quadrants and measuring approximately 15×10 cm in size. The mass was palpated distinctly from the liver. A routine ultrasound of abdomen was requested. On ultrasound (Figure 1) the mass was heterogeneously hypoechoic, with well-defined lobulated margins, central necrotic areas, and size of 13.7×10.9 cm. Linear hyperechoic areas with dirty posterior shadowing were also seen in the core of the lesion, suggestive of air. On colour Doppler study, the lesion demonstrated both arterial and venous vascularity. To confirm if the origin of air foci within the lesion was from the bowel loops, a small bowel series was done.
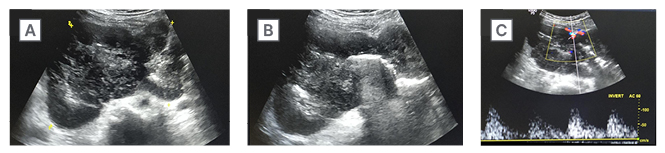
Figure 1: Ultrasonography images.
A) These show a well-defined, heterogeneously hypoechoic, lobulated solid lesion, with central necrotic areas.
B) There are linear hyperechoic areas with dirty posterior acoustic shadowing in the core of lesion, suggestive of luminal air.
C) Colour doppler shows vascularity within the lesion.
On pre-contrast radiograph (Figure 2A), there was a large abnormal soft tissue opacity of size 15×10 cm in the umbilical and right lumbar quadrant, with a central lucent area. On small bowel series (Figure 2B), gastric distensibility and emptying was normal. The core of the mass showed faint contrast opacification. The mass had displaced the ileal loops to the left. To radiologically confirm the diagnosis, and plan surgical resection of the mass, a contrast-enhanced CT (CECT) abdomen with oral contrast was performed. Axial sections of the CECT abdomen (Figure 3A) showed a large, well-defined, heterogeneously enhancing, lobulated exophytic mass, with the central necrotic area arising from the small bowel loops occupying the umbilical and both lumbar quadrants. The central core of the lesion demonstrated contrast filling with air-contrast level. There were no calcifications. There were no enlarged surrounding mesenteric or retroperitoneal lymph nodes. Under ultrasound guidance, the lesion was biopsied.
Histopathology (Figure 3B) revealed spindled shaped cells arranged in sheets, whorls, and vague storiform pattern. Individual spindle cells had ovoid to elongated, blunt ended pleomorphic nuclei, with prominent nucleoli. The cells also showed a moderate amount of fibrillary eosinophilic cytoplasm and vacuolations. There were abundant blood vessels in the surrounding stroma. Histopathological findings were suggestive of a spindle cell tumour. The sample was c-KIT positive. Hence, the diagnosis of GIST was confirmed. The patient was given imatinib after the surgical excision of the mass.
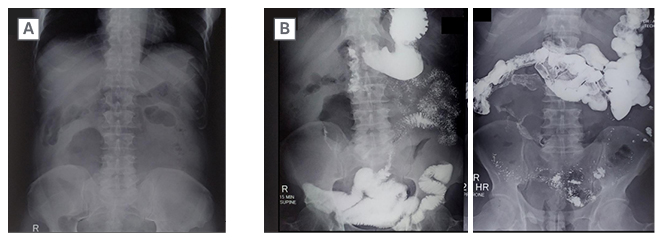
Figure 2: Pre-contrast radiograph of the abdomen.
A) Reveals a large soft tissue radio-opacity of approximate size 15×10 cm in right lumbar and umbilical quadrants with a central lucent area.
B) Small bowel series show normal gastric distensibility and emptying. There is faint opacification in the core of lesion. The mass is displacing ileal loops to the left.
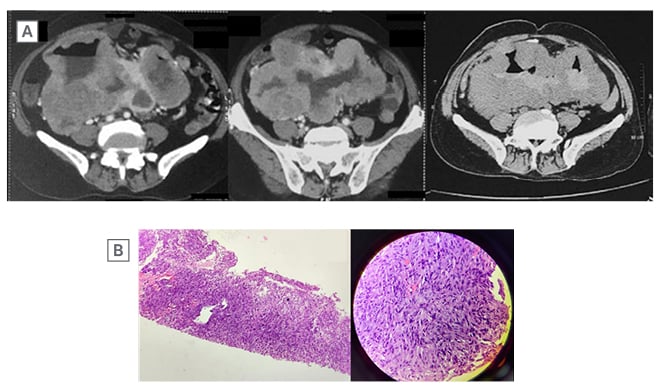
Figure 3: Axial sections of contrast-enhanced CT abdomen.
A) Shows a large, well-defined, heterogeneously enhancing, lobulated exophytic mass, with central necrotic area, arising from the small bowel loops and occupying the umbilical and both lumbar quadrants. There is contrast filling within core of the lesion with air contrast level.
B) On histopathology, there are spindled shaped cells arranged in sheets, whorls, and vague storiform pattern. Individual spindle cells have ovoid to elongated, blunt-ended pleomorphic nuclei with prominent nucleoli. The cells also show moderate amount of fibrillary eosinophilic cytoplasm and vacuolations. There are abundant blood vessels in the surrounding stroma.
DISCUSSION
Nearly half of the benign tumours in the stomach and small bowel are mesenchymal in origin, arising from the muscularis propria, and 90% of them are GIST. Other submucosal lesions are lipomas, haemangiomas, neurofibroma, inflammatory fibroid polyps, Brunner’s gland hamartoma, ectopic pancreatic rests, and duplication cysts.3,4 The most common site for GIST is the stomach in two-thirds of cases, followed by small bowel in 30% of cases. Other rare sites are the colon, oesophagus, appendix, and extra GI (omentum, mesentery, and retroperitoneum).2,5-7 Extra-GI stromal tumours are similar to GIST with respect to histology and immunophenotype; however, they are more aggressive, and similar to small intestinal GIST.7,8 Patients with GIST present with vague abdominal pain, distention, or bleeding (haematemesis, malaena, haematochezia). Other rare presentations are obstructive jaundice (duodenal GIST), and bowel obstruction (due to endophytic growth causing intussusception or lumen narrowing, or mesenteric growth causing volvulus).2,5,6
Histologically, GIST are classified into three types: spindle, epithelioid, and mixed pattern, of which the spindle variety is more common.2,9 Eighty-five percent of GIST are associated with KIT gene mutation, while 5–10% of cases are due to platelet-derived growth factor receptor A (PDGFRA) mutation.2,10 A product of the c-KIT gene, CD117, is present on the interstitial cells of Cajal, which helps to differentiate GIST from other mesenchymal tumours.6,8 The basic pathology behind the development of GIST mutation in the KIT or PDGFRA gene, leads to increased tyrosine kinase activity and unchecked growth on the tumour. To combat this, various targeted molecular therapies have been approved for GIST, such as imatinib.11,12 Imatinib competitively inhibits KIT, BCR-ABL, and PDGFRA or B.2,9,11,12 Others, like sunitinib and regorafenib, are also in clinical use for patients with GIST who are resistant to imatinib therapy, or have recurrence of disease or metastasis.5
The single-most prognostic factor to determine the malignant potential is the tumour size; however, GIST of any size should not be considered as a benign entity.9 Other features are the location of tumour in the bowel, and high mitotic activity.5,11 Endoscopic ultrasound helps to determine the layer from which the submucosal lesions originate, apart from its endoscopic findings.12-15 However, exophytic lesions are not easily detected on endoscopy; in such cases, transabdominal ultrasound is the first modality to diagnose the GIST. Plus, core particles obtained from transabdominal ultrasound-guided biopsy are superior to endoscopic ultrasound-guided biopsy to assess the mitotic index, and determine risk for malignant potential.13 On transabdominal ultrasound, GIST are well-defined hypoechoic masses with smooth or lobulated margins. Small tumours are round while large tumours are oval or ovoid. Echogenicity is similar to that of the muscular layer of stomach or bowel.4,16,17
Two patterns are frequently encountered on ultrasound. Homogeneous solid mass is the most common pattern, and has low mitotic activity. Another pattern is heterogenous mass, with a central area of low echogenicity, which corresponds to fluid filled cavities of blood or necrosis.14 These tumours are usually large, and have high mitotic activity. Sometimes a fistula develops due to mucosal erosion in the central necrotic cavity of large tumours. They appear as linear hyperechoic areas with dirty post acoustic shadowing. If the tumour bleeds, acute haematomas are hyperechoic. Metastatic or advanced GIST post-treatment with tyrosine kinase inhibitors, typically have cystic appearance.18
On small bowel series, endophytic lesions show intraluminal defects. Exophytic lesions, unless large, are difficult to detect; they show mild luminal protrusion, and displacement of the adjacent bowel loops.3 Rarely, calcification is seen, showing irregular streaks or clumps of mottled calcification (mucinous adenocarcinoma of the stomach shows punctate calcification).3 Areae gastricae is normal in GIST, while it is obliterated in the mucosal disease.3 On profile view, barium study with the patient in upright position shows that the lesion has smooth margins, forming an obtuse angle with the GI wall, and a barium stalactite is seen draping down from the inferior surface.19 The same barium stalactite when viewed en face appears like a central-filled cavity within a lobulated submucosal lesion, due to ulceration. The ulceration or cavitation within the mass has direct communication with the bowel lumen. This characteristic appearance is described as target or bull’s eye lesions.2,3
Exophytic lesion shows a central dimple or spicule at the apex of mass, as a result of traction in the gastric or bowel wall by the base of mass, thus helping to differentiate it from the extrinsic lesion.3 Findings on CT (the primary modality of choice for GIST): small GIST (<5 cm) show homogeneous enhancement and are round in morphology, while large GIST (>5 cm) are lobulated with heterogeneous enhancement, and are associated with degenerative changes, like necrosis, haemorrhage, and very rarely, calcification and metastasis as well.2,6,19,20
Irrespective of the tumour size, there is mucosal ulceration which leads to GI bleeding. Cavitation is also seen in GIST, which leads to air entrapment in the necrotic ulcer (Torricelli–Bernoulli sign). Necrosis and ulceration are common as it outgrows the blood supply, or if the endophytic growth causes thinning and stretching of the overlying mucosa layer.3 Gastric GIST shows an intermediate pattern of enhancement, while small bowel GIST shows marked arterial phase enhancement. ‘Tumour vessel’ sign, a feature of small bowel GIST due to early venous return and prominence of the draining vein, helps in tracing the origin of the tumour. A nodule within the mass is a sign of recurrence.1 Treatment of choice for resectable GIST is surgery, although neoadjuvant imatinib is given to reduce the tumour size, and post-surgery imatinib is administered to prevent recurrence of the disease for at least 3 years. In case of metastasis or recurrence, imatinib is a first line drug.4,10,11,20
CONCLUSION
Although CT is the modality of choice for GIST, it is imperative for a radiologist to be well-acquainted with various other non-cross section imaging, such as radiographs, small bowel series, and ultrasound, to increase the level of confidence in radiological diagnosis before histopathology and immunohistochemistry confirms it.







