Abstract
Background: Thrombocytopenia (TCP) is the second most common haematological finding in pregnancy next to anaemia. It carries a risk for both the mother and the fetus, associated with substantial maternal or neonatal morbidity and mortality. However, a specific therapy, if instituted promptly, improves the outcome for affected patients and their offspring. In patients in India, TCP during pregnancy is an underexplored condition.
Objectives: To assess the aetiology of TCP in pregnancy and to assess the maternal outcomes of TCP in pregnancy.
Methodology: The authors included a total of 133 patients in their third trimester (>32 weeks), with a platelet count <149,000 /mm3, admitted to the authors’ institution from 1st January 2021 to 31st December 2021. Patient-related data such as menstrual and obstetric history, presenting complaints, obstetric examination, and basic investigations were collected in a pre-designed, pre-tested proforma. All cases were followed until delivery to record any maternal complications, or any other morbidities. The data were analysed using SPSS (International Business Machines Corporation, Armonk, New York, USA) software. χ2 test was used to compare the proportions between the groups. p<0.05 was considered significant.
Results: Overall, 64.7% of patients were in the 18–25 years age group and 49.6% of patients were primigravida. Furthermore, 60.9% of patients were diagnosed to have mild TCP, 32.3% had moderate TCP, and only 6.8% patients had severe TCP. The majority (75.2%) of cases were of gestational TCP. In total, 15.8% of cases had pregnancy-induced hypertension (PIH); 3.0% had dengue; 2.3% were COVID-19 positive; 1.5% were diagnosed with haemolysis, elevated liver enzymes, and low platelets syndrome; 1.5% had immune TCP; and only one patient had leptospirosis. Four percent of cases had gestational TCP, 9.5% had PIH, one patient (25.0%) had dengue, and both cases of immune TCP had severe TCP. Twenty-eight percent of gestational TCP cases; 47.6% of PIH cases, both cases of haemolysis, elevated liver enzymes, and low platelets syndrome; 50.0% of dengue cases; and one COVID-19 positive case (33.0%) had moderate TCP. Finally, 6.25% of patients who underwent lower segment caesarean section had severe TCP, 6.00% of patients who underwent vaginal delivery had severe TCP, and out of two patients who had a spontaneous abortion, one (50.00%) had severe TCP at the time of admission. The association was significant (p<0.05).
Conclusion: TCP is a crucial condition among pregnant patients. Mild TCP is a common type. Correct aetiological diagnosis, and promptly administered adequate and specific therapy are, therefore, essential to significantly improve the outcomes of pregnant patients and their offspring.
Key Points
1. During pregnancy, the second most common haematological complication after anaemia is thrombocytopenia (TCP). This coagulation disorder causes an abnormally low level of platelets.2. The authors’ studied cohort of patients in their third trimester with a platelet count <149,000 /mm3 had predominantly mild cases of TCP, with the majority of cases due to gestational TCP.
3. TCP is associated with heightened maternal or neonatal morbidity and mortality, but the implementation of specific treatments, tailored to aetiology, can significantly improve outcomes.
INTRODUCTION
Thrombocytopenia (TCP) is a common condition that occurs in pregnancy. It is the second most common haematological finding in pregnancy next to anaemia. In pregnancy, physiological and pathological changes occur in platelet number and their functions, which can be of clinical concern. Inherited qualitative and quantitative platelet disorders may also manifest during pregnancy with the risk of bleeding.1
TCP affects 7–10% of all pregnancies.1 Most studies report a reduction in platelet count approximately 10% lower than pre-pregnant values. The normal range of platelets in non-pregnant patients is 150–400×109 /L.1 TCP is defined as a drop in platelet count below 150×109 /L. It may result from a variety of causes, from a spectrum of benign conditions such as gestational TCP, to life-threatening syndromes such as haemolysis, elevated liver enzymes, and low platelet (HELLP) syndrome. Gestational TCP is the most common aetiology, which accounts for almost three-quarters of all cases. TCP complicating hypertensive disorders of pregnancy are responsible for approximately 20% of all cases of TCP during pregnancy. Gestational or incidental TCP, the most common cause of TCP during pregnancy, accounts for 75% of pregnancy-associated TCPs. The incidence of immune thrombocytopenia (ITP) is one case of TCP per 1,000 pregnancies, and it accounts for 5% of cases of pregnancy-associated TCP.2 The authors found significant TCP most commonly in the first trimester. Pre-eclampsia is present in 21% of cases of maternal TCP.3 TCP occurs in 50% of patients with pre-eclampsia, and occasionally precedes other manifestations of the disease.
TCP is very risky for the mother and the fetus, and it is associated with maternal or neonatal morbidity, as well as mortality. If a specific therapy is applied, it will improve the outcome for affected patients and their offspring.4 There is limited research that investigates platelet count during pregnancy in India. This study was conducted to assess causative aetiology and outcome of TCP in pregnancy in India.
MATERIAL AND METHODS
The study was conducted in the department of Obstetrics and Gynaecology, Dr. D. Y. Patil Medical College Hospital and Research Institute, Kolhapur, India. This study was a hospital-based, prospective, observational study. A total of 133 patients who were admitted in their third trimester (>32 weeks) took part in the study. The sample was calculated on the basis of the Cochran formula (4 pq/L2) on the basis that TCP affects 7–10% of pregnancies.1 Patients were selected by the universal sampling method. The study was conducted from 1st January 2021 to 31st December 2021.
Inclusion criteria to enrol in the study were antenatal patients with platelet count lower than 150×109 /L, in the third trimester, and who were willing to participate in the study. Exclusion criteria were patients who refused participation; patients with known haematological disorders; patients with a history of diabetes, collagen disorders, tuberculosis, and epilepsy; and patients who had had a previous caesarean section.
Approval from the Institutional Ethics Committee (IEC) was received. Informed consent from the patients and patient-related data were collected, including detailed menstrual and obstetric history; presenting complaints; and findings of general, systemic, and obstetric examination, including pelvic examination. Investigations were performed, including urine tests for albumin/sugar, complete blood count, liver function test, renal function test, peripheral blood smear, coagulation profile, detection of malaria, and collection of dengue IgG and IgM antibodies, in a pre-designed, pre-tested proforma. Gestational age was established by menstrual history and clinical examination, confirmed by ultrasonography. Platelet count of 100,000–149,999 /mm3 was classified as mild TCP; 50,000–100,000 /mm3 as moderate TCP; and <50,000 /mm3 as severe TCP.
All the cases were followed until delivery to record any complications such as pre-term labour, abruption, pre-eclampsia, postpartum haemorrhage, or any other morbidities. Duration of pregnancy at the time of delivery, indication of induction, and method (if required) and mode of delivery, including indication for instrumental delivery or caesarean section, were recorded. The data were coded and entered in Microsoft Office Excel 2020 (Microsoft, Redmond, Washington, USA). The data were then analysed using SPSS software version 21 (International Business Machines Corporation [IBM], Armonk, New York, USA). The categorical data were represented as a number (percentage) and numerical data as mean with standard deviation. An χ2 test was used to compare the proportions between the groups for samples larger than 30. Mann–Whitney U test was used to compare the means of quantitative data with the non- normal distribution. P<0.05 was considered statistically significant.
RESULTS
In the present study, 133 pregnant patients with confirmed cases of TCP were enrolled. Demographic characteristics of the cohort are displayed in Table 1. Most of the patients (86 [64.7%]) were in the 18–25 year age group, followed by 34 patients (25.6%) from 26–30 years. The majority (107 [80.5%]) were homemakers, followed by eight teachers, seven nursing staff, and six laboratory technicians. Furthermore, 130 (97.8%) were Hindu, 64 (48.1%) were from the lower-middle class, and only one patient was illiterate. Parity shows that 66 (49.6%) patients were primigravida and the remaining 67 (50.4%) were multipara (Table 1).
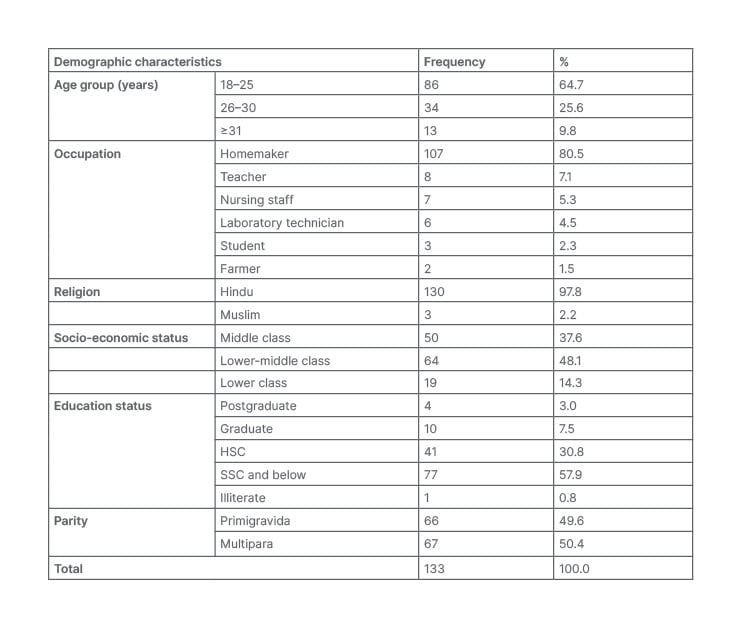
Table 1: Demographic characteristics of the patients.
HSC: higher secondary certificate; SSC: secondary school certificate.
Based on platelet counts on admission, patients were categorised into three groups: mild, moderate, and severe TCP. Figure 1 illustrates the distribution of TCP by severity. In total, 81 (60.9%) patients were diagnosed to have mild TCP (1 Lakh–1.5 Lakh), 43 (32.3%) moderate (50,000–1 Lakh), and only nine (6.8%) patients were found to have severe TCP (≤50,000 [ Figure 1 ]).
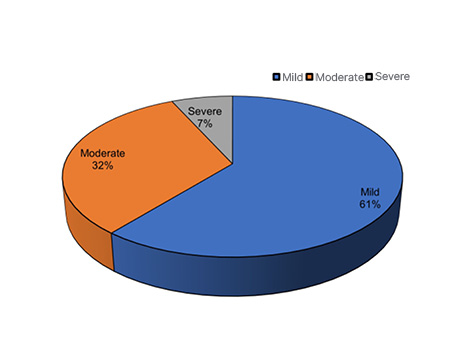
Figure 1: Grades of thrombocytopenia.
Figure 2 demonstrates the prevalence of each underlying aetiology of TCP. The majority (100 cases [75.2%]) were of gestational TCP. Further, 21 (15.8%) cases had PIH; four (3.0%) had dengue; three (2.3%) were COVID-19 positive; two (1.5%) were diagnosed with HELLP syndrome; two (1.5%) had ITP; and only one patient had leptospirosis.
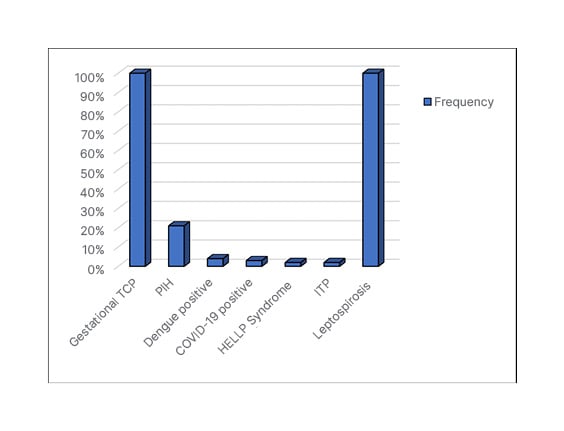
Figure 2: Aetiology of thrombocytopenia.
HELLP: haemolysis, elevated liver enzymes, and low platelets; ITP: immune thrombocytopenia; PIH: pregnancy-induced hypertension; TCP: thrombocytopenia.
Table 2 shows an association between various aetiologies and maternal outcomes with grades of TCP. Among aetiologies, four (4.0%) cases of gestational TCP, two (9.5%) cases of PIH, one case (25.0%) of dengue, and two cases of ITP had severe TCP. Furthermore, 28 (28.0%) cases of gestational TCP, 10 (47.6%) cases of PIH, two cases of HELLP syndrome, two (50.0%) dengue cases, and one COVID-19 positive case (33.0%) had moderate TCP. The remaining cases had mild TCP. The association was found to be significant (p<0.05).
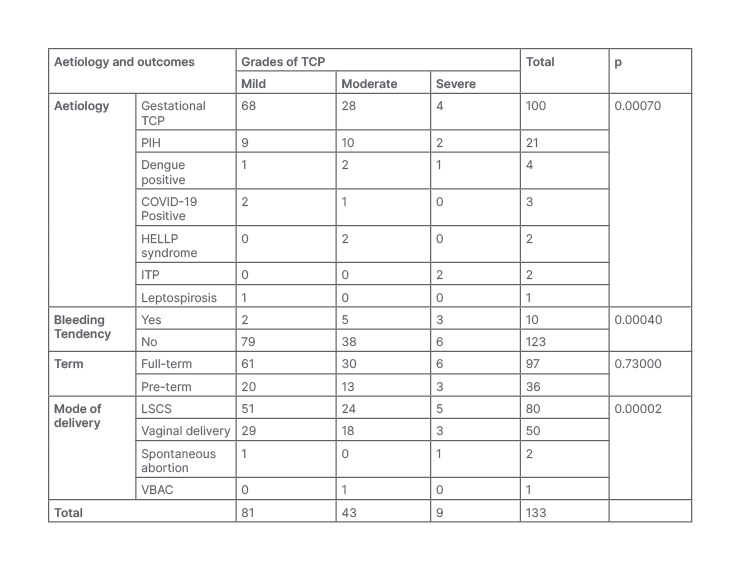
Table 2: Association of aetiologies and maternal outcomes with grades of thrombocytopenia.
ITP: idiopathic thrombocytopenic purpura; HELLP: haemolysis, elevated liver enzymes and low platelet; LSCS: lower segment caesarean section; PIH: pregnancy-induced hypertension; TCP: thrombocytopenia; VBAC: vaginal delivery after caesarean.
Three (30%) patients with bleeding tendency had severe TCP, but only six (5%) of the patients without bleeding tendency had severe TCP. The association was significant (p<0.05). Sixty-one full-term patients had mild TCP versus only 20 pre-term patients. The bleeding tendency was higher in patients with severe TCP but the difference was not significant (p=0.73).
Five (6.25%) patients who had undergone lower segment caesarean section (LSCS) had severe TCP, three (6.00%) patients who undergone vaginal delivery had severe TCP; and out of two patients who had a spontaneous abortion, one (50.00%) had severe TCP at the time of admission. There were more complications in patients with severe TCP and the association was significant (p<0.05). After admission, every patient was managed and platelet counts were achieved. Later the patients delivered as per the indications.
DISCUSSION
In the authors’ study, of the total 133 cases studied, the majority (75.2%) were found to have gestational TCP; 15.8% cases had PIH; 3.0% had dengue, 2.3% were COVID-19 positive; 1.5% were diagnosed to have HELLP syndrome; 1.5% had ITP; and only one patient had leptospirosis. In a study conducted by Wang et al.,5 the incidence of gestational TCP was 60.0%, while the incidence of hypertensive disorders was 28.2%, and other causes, including ITP, made up 11.8%. The results in the authors’ study are comparable with this study.
In a study conducted by Sainio et al.,6 the incidence of gestational TCP was 81%, while that of pre-eclampsia was 16%, and ITP was 3%. This study included only term patients, and this may be the reason for a slightly higher incidence of pre-eclampsia in the authors’ study.
A study conducted by Parnas et al.7 found that the main causes of TCP were gestational TCP (59.30%), immune thrombocytopenic purpura (11.05%), pre-eclampsia (10.05%), and HELLP syndrome (12.06%). In study conducted by Anita et al.,8 gestational TCP included 64%, with hypertensive disorders making up 21%, and other disorders 13%. Ajzenberg et al.9 assumed that gestational TCP occurs due to increased platelet consumption within the placental circulation and/or normal inhibition of megakaryocytopoiesis.
During pregnancy, haemodilution caused by the relative increase in plasma volume, coupled with increased platelet turnover, leads to the development of so-called gestational or incidental TCP, which accounts for three-quarters of cases of TCP detected during pregnancy.10
Immune-mediated TCP is an autoimmune disorder caused by the development of IgG auto-antibodies that are directed against a number of platelet glycoproteins. Antibody-bound platelets are rapidly cleared from the maternal circulation once they bind specific antibody receptors on macrophages, which might cause TCP.10
There is endothelial cell activation or dysfunction in pre-eclampsia. Cytokines such as TNF-α and ILs may contribute to the systemic oxidative stress associated with pre-eclampsia. This is characterised by reactive oxygen species and free radicals, which lead to the formation of self-propagating lipid peroxides. These peroxides in turn generate highly toxic radicals that injure systemic vascular endothelial cells, modify nitric oxide production by these cells, and interfere with prostaglandin balance. Other consequences of oxidative stress include the production of the lipid-laden macrophage foam cells seen in placental atherosis, and the activation of systemic microvascular coagulation manifested by TCP.11 Burrows and Kelton12 proposed that, in addition to an increased vascular tone during pregnancy, inducing platelet destruction, coagulation defects also occur. Some patients who are pregnant and have hypertension have an increased platelet-related IgG serum level; however, the increase in Ig is not specific, and does not necessarily indicate an immunologic-mediated TCP.13 All patients with HELLP syndrome may have an underlying coagulopathy, which is usually undetectable. TCP has been attributed to increased consumption and/or destruction of platelets.14
In dengue infection, the dengue virus could directly or indirectly affect bone marrow progenitor cells by inhibiting their function to reduce the proliferative capacity of haematopoietic cells.15 Increased platelet clearance may occur in dengue infection as a consequence of platelet activation. The platelets from patients with dengue display classic signs of apoptosis that include increased phosphatidylserine exposure, mitochondrial depolarisation, and activation of caspase-9 and 3. Moreover, TCP in patients with dengue strongly associates with enhanced platelet activation and apoptosis.16 COVID-19 may increase levels of auto-antibodies and immune complexes, resulting in specific destruction of platelets by the immune system.17
In the present study, most of the females were between 18–25 years. Of those patients, 49.6% were primigravida and 50.4% were multigravida. Asrie et al.4 conducted a study in Ethiopia in 2014, which reported that 35% of the study group were primigravida and 65% were multigravida. There were more primigravida patients in the authors’ study compared with this study.
In the authors’ study, among those with gestational TCP, 68% had mild TCP, 28% had moderate TCP, and 4% reported severe TCP. This shows that gestational TCP is usually of mild severity. This is comparable with Olayemi et al.’s18 study in Ghana, where 65% had mild TCP. Boehlen et al.19 also reported that gestational TCP is usually mild.
The present study shows that two (9.5%) cases of PIH had severe TCP; and 10 (47.6%) cases of PIH and both cases of HELLP syndrome had moderate TCP. The results are comparable with the study conducted by Rupakala et al.20 on TCP in hypertensive disorders in pregnancy, in which severe TCP is seen in 5.8%, moderate TCP in 35.5%, and mild TCP in 58.7% of cases. Incidence of HELLP in this study was 6.60%, which is also comparable to the authors’ study. Özdemir et al.21 showed that the incidence of TCP in patients with hypertension was 24.2% and severe TCP was seen in 8.1% of cases. Magann et al.22 reported that severe TCP is seen in 12% of patients with HELLP syndrome, 30% of eclampsia cases, and 18% of severe pre-eclampsia cases.
In the authors’ study 60.0% of patients underwent a LSCS, 38.0% delivered vaginally, two patients (1.5%) had a spontaneous abortion, and one case delivered vaginally after a caesarean section. Further, 6.25% of patients who underwent a LSCS had severe TCP, 6.00% of patients who underwent a vaginal delivery had severe TCP, and out of two patients who had a spontaneous abortion, one (50.00%) had severe TCP at the time of admission. In a study at Safdarjung Hospital, New Delhi, India, it has been found that around 94.00% patients delivered vaginally and, among these, nine patients had instrumental delivery.23 In the authors’ study 38.00% of patients delivered vaginally, which is lower when compared with this study. The American College of Obstetricians and Gynecologists (ACOG) recommends that the definitive treatment of maternal TCP in the setting of PIH with HELLP syndrome is the termination of pregnancy.
Activation of the coagulation and fibrinolytic systems cause the development of life- threatening TCP and disseminated intravascular coagulation, which occurs in some patients having symptoms of pre-eclampsia, and plays a role in stimulating platelet activation and accelerated clearance.24 The authors came to the conclusion that HELLP syndrome causes haemolysis, liver function disturbance, lower count of platelets, and severe hypertension in patients, and that they are associated with high maternal and fetal morbidity, as well as mortality.
CONCLUSION
The most common cause of TCP in pregnancy is gestational. TCP is a crucial problem among patients who are pregnant, with mild TCP being the most common type. Correct aetiological diagnosis, and promptly administered adequate and specific therapy are therefore essential to significantly improve the outcomes of pregnant patients and their offspring; therefore, specific attention should be given to patients with severe TCP due to pre-eclampsia and HELLP syndrome to establish the best moment for therapeutical intervention.







