Abstract
In vitro fertilisation (IVF) with donated oocytes is the most effective assisted reproduction treatment currently available; however, repeated implantation failure (RIF) can occur with this treatment. The protocol of patient preparation for IVF with donated oocytes is relatively simple and works well in most cases; however, it can fail in a minority of women, which is what occurs in RIF patients. While the probability of RIF occuring is 20–35%, it is reasonable to take adequate measures in all patients in order to avoid procedural failure. The risk of oocyte donation failure can be minimised by applying a customised oocyte donation enhancement (CODE) protocol, in which pitfalls of the standard protocol are detected and corrected in the pretreatment phase, during the patient’s uterus preparation for embryo transfer, and after the transfer. Growth hormone, recently reported to improve pregnancy outcomes in women with RIF after oocyte donation, is a possible component of the CODE protocol, but it cannot be considered a unique solution to RIF. This article reviews possible causes of RIF and places growth hormone treatment in the context of other important measures to be followed in the CODE protocol.
INTRODUCTION
Assisted reproduction with oocyte donation has very high success rates; if performed with fresh oocytes from donors <26 years of age, the probability of live childbirth is nearly 75%.1 The probability of a live birth is slightly lower with older oocyte donors (within the age limits imposed by the law of each country in which oocyte donation is performed, usually around 35 years) or when cryopreserved oocytes are used.2
A question arises of what happens in those 25% of women who fail to become pregnant or who miscarry after oocyte donation. Sometimes it is unexplained and the patient becomes pregnant on the next attempt, which can be completed with spare cryopreserved embryos from the first attempt in most cases. However, there are cases of repeated failures of assisted reproduction with oocyte donation. The author has reported recently that growth hormone (GH) can be of help in some of these cases.1 In the present paper, the most common causes of implantation failure in oocyte donation programmes are discussed. A strategy of customised oocyte donation enhancement, aimed at avoiding all potential pitfalls responsible for these failures, is outlined, with particular attention to the indications for GH administration to donor oocyte recipients.
CURRENT STATUS OF OOCYTE DONATION
Since the first report of a term pregnancy using in vitro fertilisation (IVF) with donated oocytes in a patient with primary ovarian failure,3 oocyte donation has gained traction in the treatment of infertility in many other conditions,4 including young amenorrhoeic women with premature menopause5,6 as well as naturally menopausal women,7 repeated IVF failures,8,9 and women with a genetic trait precluding the use of their own oocytes.4,9 When performed with fresh oocytes from donors <26 years of age, the ongoing pregnancy rate can be as high as 75%,1 the highest success rate among all other assisted reproduction treatment options available today. However, there remains an important question: what can be done for women who fail to become pregnant with this procedure? Is it merely a case of bad luck, or is there a factor that can be examined, determined, and treated? In the experience of the author, many of the women who fail to become pregnant with the first instance of oocyte donation succeed with the next attempt. However, some women experience repeated failures during subsequent attempts. This group of patients has a critical need for an individualised approach to achieve their goal.
Success Rate
The aforementioned 75% success rate can be considered optimal for the procedure, but it is not currently achieved in all oocyte donation programmes worldwide. In fact, real-world success rates reported by different IVF centres tend to be lower than this, especially when cryopreserved oocytes are used.2 Oocyte donation attempt failure should not be automatically explained as ‘bad luck’; in these cases, a systemic error is a more probable explanation. As with all systemic errors, the cause can be searched for, identified, and an appropriate solution can be devised. To make this possible, it is important to define the factors that can compromise the chances of success for couples seeking this kind of treatment.
Pitfalls
The cause of oocyte donation failure can be determined through careful examination in most cases; however, it is mandatory to follow the evolution of the patient’s response to the treatment, both before and after embryo transfer, and to detect abnormalities compared with the normal course of events, in every case. Moreover, the possibility of implied male-borne factors should not be neglected. Consequently, possible causes of oocyte donation failure should be searched for, and corrected if necessary, even before the patient’s inclusion in the programme.
CAUSES OF OOCYTE DONATION FAILURE
An oocyte donation attempt can fail because of female factors, male factors, or both. The female factor tends to be underestimated in the context of oocyte donation. The reason for this is that poor oocyte quality is the most common condition causing female factor infertility and IVF failure when using the patient’s own oocytes; however, one problem could mask another, and the fact that the most probable cause of the patient’s infertility has been taken into account does not exclude the presence of other, less frequent but no less important, problems. The probability of the male factor is the same as in IVF with the patient’s own oocytes.
Female Origin
Problems of female origin can be detected before treatment initiation, during the treatment for endometrial preparation for embryo transfer, or in the period following embryo transfer.
Morphological Abnormalities of the Uterine Endometrial Cavity
Endometrial polyps, adhesions, and protruding leiomyomas are the most commonly found morphological abnormalities of the uterine cavity, and they can be found in women with a seemingly normal uterus. If not diagnosed and adequately treated, these conditions can lead to repeated failures of assisted reproduction with donor oocytes. These pathologies can be easily detected by hysteroscopy; however, this is not always carried out before starting an assisted reproduction attempt because it may result in discomfort for the patient when conducted without anaesthesia, but using anaesthesia increases the cost of the procedure. The recently developed technique of virtual sonographic hysteroscopy,10 which enables a more patient-friendly approach and avoids the need for anaesthesia, may be used in the future for all cases of IVF with donated oocytes to avoid unexpected failures due to uterine cavity abnormalities. Conventional operative hysteroscopy is, thus, only performed in women in whom abnormalities of the uterine cavity have been detected by this preliminary examination (Table 1).
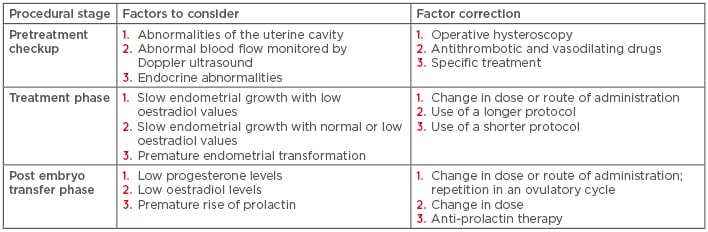
Table 1: Customised protocol of endometrial preparation for oocyte recipient patients showing where and how pitfalls can be detected.
Abnormal Blood Flow
Endometrial and subendometrial blood flow distribution pattern is correlated with the implantation and pregnancy rate after IVF treament.11 This condition can be easily assessed by transvaginal colour Doppler ultrasound in the pretreatment phase and efficiently resolved by the administration of vasodilating drugs, such as pentoxifylline,12 during the preparatory treatment of oocyte recipient women (Table 1).
Endocrine Disorders
Hyperthyroidism, hypothyroidism, hyperprolactinaemia, and insulin resistance can all negatively affect the outcome of IVF (Table 1). Here again, the possibility of one problem masking another must be discussed to avoid the temptation of oversimplifying procedural failure by blaming oocyte quality for all previous IVF failures, without taking into consideration other possible contributing factors.
Systemic Disorders
Systemic factors that can contibute to IVF failure in oocyte donation cycles include congenital or acquired thrombophilia, resistance to the activated protein C, lupus anticoagulant, and anticardiolipin antibodies.13
Abnormal Evolution of Endometrial Thickness and Morphology
It is generally assumed that successful implantation after IVF is less frequent in women who do not reach adequate endometrial thickness at the time of embryo transfer. The pregnancy rate in women with endometrial thickness <7 mm is lower compared to those with endometrial thickness ≥7 mm;14,15 however, some patients prepared for IVF with donated oocytes do not achieve this value easily. There may be different factors causing this condition, among which an abnormal metabolism of exogenous steroid hormones is the most common.
Abnormal Metabolism of Exogenous Steroid Hormones
Patients receiving embryos originating from donated oocytes are usually treated with exogenous oestradiol and progesterone to ensure an adequate preparation of their endometrium for embryo implantation. If embryos are transferred during a natural ovulatory cycle, this treatment can, in theory, be avoided; however, this is not possible in cases of fresh oocyte donation, wherein the cycles of the oocyte donor and recipient have to be synchronised, in menopausal women, and in those with premature ovarial failure who lack ovulatory cycles.
There are different possible methods of oestradiol and progesterone administration. Oestradiol is most commonly administered orally or transdermally. When taken orally, most oestradiol is converted to less biologically active metabolites, oestrone and oestriol, in the liver during first-pass metabolism.16 Transdermal application can avoid hepatic passage and prolong the active life of oestradiol;17 however, vaginal administration of oestradiol has proved to be the most efficient method.18 Progesterone can be administered orally, vaginally, or via intramuscular injections. Orally administered progesterone is rapidly metabolised in the gastrointestinal tract; oral administration has proved to be inferior to intramuscular and vaginal routes.19 Significant differences in serum oestradiol and progesterone concentrations were observed between patients with the use of standardised protocols, leading to a need for repeated determinations during the treatment procedure.20,21
Male Origin
Oocyte donation cannot resolve problems of sperm quality,20,21 although some consequences, especially those related to sperm DNA fragmentation, can be corrected by healthy oocyte cytoplasm.22
CUSTOMISATION OF PROTOCOLS FOR PATIENTS RECEIVING DONOR OOCYTES
Similar to ovarian stimulation protocols in women with poor ovarian reserve,23 protocols for donated oocyte recipient can be customised before starting the treatment, during patient preparation for embryo transfer, and after embryo transfer.
Pretreatment Phase
Correction of Endometrial Pathologies
Different endometrial pathologies can be detected by virtual sonographic hysteroscopy, based on a simple vaginal ultrasound scan using Fly-Thru technology (Toshiba, Tokyo, Japan).10 Owing to its patient-friendly nature compared to conventional hysteroscopy, virtual hysteroscopy can be performed in all potential oocyte donation patients to provide details of any problems that might contribute to treatment failure. Conventional operative hysteroscopy would then be performed only in women in whom virtual hysterosopy shows a relevant pathology that needs to be corrected.10
Abnormal Blood Flow
When subendometrial uterine blood flow determined by Doppler sonography is abnormal, treatment with vasodilating drugs, such as pentoxifylline, should be considered.12,24
Specific Treatment of Eventual Systemic Pathologies
When detected, systemic disorders, such as thrombophilia, and resistance to the activated protein C, lupus anticoagulant, and anticardiolipin antibodies should be treated accordingly using anticoagulants and corticosteroids.
Treatment of the Male Factor
Sperm DNA fragmenation, the most common male cause of IVF failure, can be alleviated in different ways.22 Prolonged (2–3 months) oral antioxidant treatment (e.g., 1 g vitamin C and 1 g vitamin E daily) is sufficient in most cases. Melatonin has also been shown to protect sperm DNA against oxidative damage.25 If no improvement in sperm DNA integrity occurs following oral antioxidant treatment, other solutions, such as modifications of the IVF technique or recourse to testicular sperm, can be considered.22
Between the Start of Endometrial Growth Stimulation and Embryo Transfer
Repeated Determinations of Endometrial Thickness and Serum Oestradiol
The protocol of endometrial growth stimulation in women prepared for IVF with oocyte donation is based on treatment with progressively increasing doses of oestradiol. In most cases, oestradiol is administered orally, either mimicking the normal cycle or at a fixed dose.26 However, both kinds of protocol may result in an insufficient increase in blood oestradiol concentrations and/or the endometrial thickness. This problem can be avoided by repeated determinations of blood oestradiol and sonographic measurements of the endometrium.20 If necessary, the planned dose or administration route of oestradiol can be modified at this stage, the vaginal route being the most robust to ensure blood oestradiol increase and endometrial growth. If the endometrium grows insufficiently in spite of elevated blood oestradiol levels, vasodilating agents, such as pentoxifylline (as mentioned earlier) or vaginal sildenafil,27 can be of help. In addition to problems of oestradiol, prolactin can increase unexpectedly during patient preparation for oocyte donation, even if basal prolactin levels were normal (Tesarik J, unpublished observations). This can be easily corrected in the course of the treatment.
After Embryo Transfer
Assisted reproduction with oocyte donation is usually performed in anovulatory cycles. Consequently, there is no corpus luteum and the blood oestradiol and progesterone levels are entirely dependent on exogenous administration. In the author’s experience, blood levels of oestradiol and progesterone may fall abruptly on days following embryo transfer, even when they were normal on the day of transfer. Prolactin can also increase prematurely, requiring appropriate medication. In the author’s oocyte donation programme, oestradiol, progesterone, and prolactin levels are evaluated as early as 7 days after embryo transfer and then once a week during the first month. If no problems are detected during this period, subsequent controls are carried out every 2 weeks until the end of the first trimester of pregnancy. Vaginal progesterone supplementation is often insufficient during this period, leading to the need for intramuscular injections.
THE ROLE OF GROWTH HORMONE
In addition to its effects on oocyte quality,28-30 GH has been recently shown to have a beneficial effect on the uterine receptivity for embryo implantation.1 Experiments in a bovine model suggested an effect of GH at the uterine level; GH not only increased embryonic development in superovulated females, but it also improved post-transfer pregnancy rates when given to lactating recipient cows.31
Methods of Prescription and Dosage of Growth Hormone
GH was prescribed in oocyte recipient patients with unexplained implantation failure. Most of the patients showed a weak increase in endometrial thickness in response to oestradiol treatment during their preparation for oocyte donation. Subcutaneous injections of 1 mg recombinant human GH (Nutropin AQ®, Ipsen Pharma, Paris, France) were administed daily during 10 consecutive days of the endometrial proliferative phase induced by exogenous oral oestradiol. The treatment was adjusted individually for each patient so that the last of the 10 injections was administered 1 or 2 days before starting the treatment with vaginal progesterone to induce the secretory phase.1
Outcomes in Patients with Repeated Implantation Failure and Ensuing Questions
In a randomised controlled trial including 70 women with repeated implantation failure (RIF) after IVF with oocyte donation, patients treated with GH during endometrial growth stimulation showed a significantly thicker endometrium and higher pregnancy and live birth rates compared with RIF patients treated with placebo.1 These recent observations raise several questions; for example, would RIF have been avoided if GH had been used during previous IVF attempts? If so, how could this patient’s condition have been diagnosed? Secondly, if diagnosis is not possible at the current stage of knowledge, should GH be used in all patients receiving donated oocytes to avoid unexpected failures? Finally, could GH improve the patient’s chance of getting pregnant in other conditions, unrelated to oocyte donation?
Possible Mechanisms of Growth Hormone Action
GH is mainly secreted by the anterior pituitary gland, but it is also expressed in a variety of other tissues, including those involved in the reproductive system. GH can act via its own receptor or by means of the activation of insulin-like growth factor 1.32 The mechanism of its effect on the uterine receptivity in IVF with donated oocytes is currently unknown, but a similar improvement has also been observed in women receiving their own frozen-thawed embryos.33
Towards a Better Definition of the Target Patient Group
In the absence of conclusive data for assessing which patients will benefit from GH treatment to improve their uterine receptivity, the decision about its use remains arbitrary. GH potentiates endometrial growth and its use can, thus, be considered in women whose endometrium does not grow sufficiently with standard treatments. In view of previous observations,1 it might not be reasonable to wait for a patient to have RIF before making use of GH in the preparation of their uterus for oocyte donation. With the exception of pre-existing pathological conditions, such as certain types of tumours, postoperative and post-traumatic conditions, or acute respiratory distress, a short exposure to GH, such as that used in a recent study,1 has not been reported to bring about any important negative health effect. Consequently, the relatively elevated price of the treatment (currently €230.00 in Spain) and administrative limitations of its prescription remain the most important obstacles for its wider use. Work is in progress to design diagnostics that can identify which patients could benefit from GH treatment to improve their uterine receptivity, not only in the context of oocyte donation but also in that of IVF and natural conception in general.
DISCUSSION
Oocyte donation is a highly efficient assisted reproduction technique if its main indication is the absence or a poor quality of oocytes in the recipient patient, without other associated contributing factors. However, the efficacy of oocyte donation decreases if the fertility problem is multifactorial, and other pathological conditions of male or female origin must be taken into account.
Possible additional female factors unrelated to the status of the patient’s own oocytes, such as the presence of endometrial pathologies, abnormal subendometrial blood flow, different kinds of endocrine and other systemic disorders, and abnormalities of exogenously administered hormone metabolism, causing a deficient endometrial response in the proliferative or the luteal phase of development, are highlighted in this paper. The most common male factor causing RIF, in the current era of intracytoplasmic sperm injection as the preferred IVF technique, is excessive sperm DNA fragmentation. If detected, each of these factors can be treated adequately in the pretreatment phase, during the patient’s preparation for embryo transfer, or in the period following the embryo transfer. RIF can also occur in cases in which all effort has been made to discover the cause, but everything appeared to be normal or, if applicable, adequately controlled. It has been shown that treatment with recombinant human GH during the proliferative phase of endometrium preparation is beneficial in some cases.1 Nevertheless, there is no doubt that unexplored or poorly explored areas of research remain in this respect. Immunological incompatibility between the killer-cell immunoglobulin-like receptors (KIR) expressed in uterine natural killer cells, on the one hand, and the human leukocyte antigen-C (HLA-C), acting as KIR ligands, on the surface of the embryonic cells, on the other, are known to cause different types of pregnancy complications,34 and preliminary data suggest that HLA-C/KIR may be implicated in RIF too. Therefore, in the author’s oocyte donation programme, oocyte recipient patients are recommended to have their KIR haplotype tested. In cases of women with KIR-AA haplotype, known to be associated with an increased risk of RIF or early embryo wastage, or when the result of KIR analysis is not available at the time of the treatment attempt, an oocyte donor is chosen who does not express HLA-C2 molecules, particularly prone to KIR-mediated complications of embryo implantation and pregnancy. This cannot be done if the HLA-C2 comes from the male partner of the patient treated by oocyte donation. Different therapeutic solutions to alleviate the KIR/HLA-C incompatibility are currently under development.
CONCLUSION
Oocyte donation is a highly efficient approach to the treatment of female infertility caused by the absence of oocytes or poor oocyte quality. Failures of assisted reproduction attempts using donor oocytes are mostly due to associated pathologies, of female or male origin, which are superimposed on this main pathology for which oocyte donation is indicated. Therefore, it is important to look for any possible pathological conditions that could hinder treatment outcomes and, if detected, to treat them adequately. However, unexplained repeated oocyte donation failures occur occasionally, in spite of the exclusion or adequate treatment of these oocyte-unrelated pathologies. Recent data1 have shown that GH can be of help in some of these cases. Further research is needed to define the corresponding patient population and to explain the mechanism of GH action.








