Abstract
Infertility has become a significant problem worldwide. Multiple management options are available nowadays, which include intrauterine insemination (IUI), in vitro fertilisation (IVF), and intracytoplasmic sperm injection. IUI is one of the oldest and most popular methods. After >50 years since it was first used, IUI has evolved through various innovations but still struggles to find its place in infertility management. After the introduction of revised guidelines from the National Institute for Health and Care Excellence (NICE) in 2013, there has been a surge in the use of IVF as a primary treatment modality. The aim of this evidence-based review is to highlight the factors associated with success of IUI and to find out whether IUI can be offered as a first-line treatment option for infertile couples.
INTRODUCTION
Infertility is a disease of the reproductive system and is defined by the failure to achieve a clinical pregnancy after ≥12 months of regular unprotected sexual intercourse.1 It has become a significant global problem with a considerable physical, psychological, and social impact. According to a systematic analysis of 277 health surveys, there were around 48.5 million couples suffering from infertility worldwide in 2010. Among women 20–44 years of age, 1.9% (95% confidence interval [CI]: 1.7–2.2%) had primary infertility and 10.5% (95% CI: 9.5–11.7%) had secondary infertility.2 Assisted reproductive techniques are now considered the established treatment option for couples suffering from infertility; they embrace a wide scope of techniques of which intrauterine insemination (IUI), in vitro fertilisation (IVF), and intracytoplasmic sperm injection (ICSI) are most popular.
INTRAUTERINE INSEMINATION
IUI involves the deposition of processed semen (partner/donor) in the uterine cavity close to the time of ovulation. The rationale behind IUI is to increase the gamete density at the site of fertilisation.3 The first report of IUI was published by Cohen4 in 1962, and since then this technique has evolved through various innovations. Though the basic procedure remains the same, success rates in IUI cycles have grown from 5% to >20% per cycle with advances in stimulation protocols, cycle monitoring, timing of ovulation, semen preparation methods, and luteal phase support. IUI is a simple and easy procedure, with a short learning curve and minimal equipment requirements. Being less invasive, cheaper, and accompanied by a reduced chance of complications like multiple pregnancy and ovarian hyperstimulation syndrome in comparison to IVF/ICSI, IUI is considered the first-line treatment option. It offers less psychological burden for couples and hence has a lower dropout rate and good compliance with treatment.
Prerequisites
IUI is a Level I assisted reproduction procedure where only semen handling takes place outside of the body, and the rest of the processes involved in human conception, like ovulation, fertilisation, embryogenesis, and implantation, take place naturally. Hence, it can be considered as an extension of natural conception. The prerequisites for a couple to undergo an IUI cycle are:
- Ovulatory cycle (natural or stimulated)
- At least one patent functional fallopian tube (preferably both)
- Total motile sperm count >10 million/mL
Indications and Contraindications
IUI can be offered for a wide range of indications (Table 1).
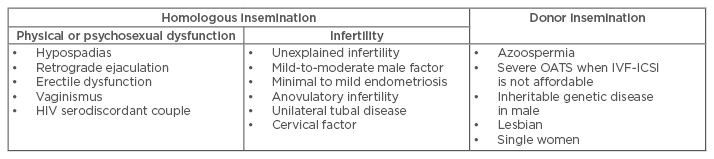
Table 1: Indications for intrauterine insemination.
IVF-ICSI: in vitro fertilisation with intra-cytoplasmic sperm injection; OATS: oligoasthenoteratozoospermia.
IUI should be avoided in a few conditions, either because it is not possible, it is less successful, or moving directly to IVF is a better option. These conditions include:
- Active pelvic infection: cervicitis, endometritis.
- Cervical stenosis, atresia.
- Blocked fallopian tubes.
- Severe oligoasthenoteratozoospermia where IVF-ICSI is the treatment of choice.
- Stage III/IV endometriosis.
- Dense pelvic adhesions.
- Poor ovarian reserve.
Prognostic Factors
The success of IUI depends upon a wide range of parameters (Table 2). In the next few sections, these parameters are discussed in light of the current evidence.
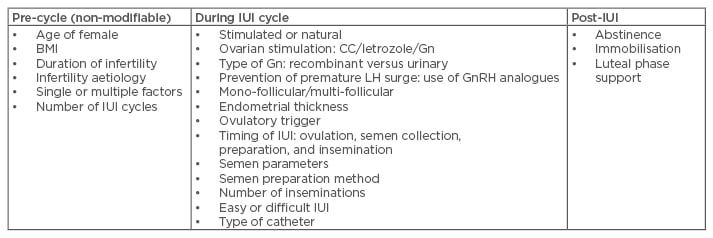
Table 2: Prognostic factors for intrauterine insemination.
CC: clomiphene citrate; Gn: gonadotropin; GnRH: gonadotropin-releasing hormone; IUI: intrauterine insemination; LH: luteinising hormone.
Age of female
Female age is the most important determinant of human conception. It is well established that clinical pregnancy and live birth rates are higher in females <35 years of age, in cases of both natural as well as assisted conception.5,6 The limited success of stimulated IUI over immediate IVF in older women was shown in the FORT-T trial.7 Hence we recommend judicious use of IUI in women >35 years of age, as declining ovarian reserve and oocyte quality may not lead to the desired results in IUI in women of advanced age.
BMI
Female obesity is an established cause for poor reproductive performance, but to date there is insufficient evidence that female obesity has an adverse effect on IUI outcomes. Further well-designed trials are therefore needed.
Duration of infertility
Most of the studies published so far have been heterogeneous in subject inclusion on the basis of infertility duration, which has typically ranged from 3–10 years. However, available evidence suggests that the longer the duration of infertility, the lower the likelihood of success with IUI.5,6
Infertility aetiology
Women with secondary infertility score better than women with primary infertility during IUI (21.4% versus 7.9%).5,8 Couples with multiple infertility factors have fewer chances of conception with IUI than couples with a single infertility factor.
Unexplained infertility
IUI in unexplained infertility has been compared in many ways. In a recent meta-analysis of 14 trials with 1,867 women, there was no difference in live birth rates between IUI and timed intercourse (TI) both in natural or stimulated cycles. However, there was an increase in live birth rate in stimulated cycle IUI in comparison to natural cycle IUI (odds ratio [OR]: 0.48; 95% CI: 0.29–0.82) without an increase in multiple pregnancy rate.9
In the FASTT trial,10 three cycles of clomiphene citrate (CC)/IUI followed by three cycles of follicle stimulating hormone (FSH)/IUI were compared with up to six cycles of IVF. In the IVF arm, there was an increase in pregnancy rate, a reduced time to conception, and lower costs per delivery.10 Similarly, the current National Institute for Health and Care Excellence (NICE) guidelines recommend IVF over IUI in unexplained infertility.11 In the recent Cochrane review,12 IVF was associated with higher live birth rates than unstimulated IUI (OR: 2.47; 95% CI: 1.19–5.12). In women pre-treated with CC/IUI, IVF appears to be associated with higher birth rates than gonadotropin plus IUI (OR: 3.90; 95% CI: 2.32–6.57). However, in treatment-naïve women there is no conclusive evidence of a difference in live birth rates between IVF and IUI with CC or gonadotropins.12
Male factor infertility
Infertility due to suboptimal semen parameters can be overcome by IUI in males in whom sperm count, motility, or morphology is affected moderately, while IVF-ICSI is the treatment of choice in cases where a severe defect or a combination of defects is present. In the two Cochrane reviews conducted on male subfertility, IUI was not better than TI in a natural cycle or stimulated cycle.13,14 Also, there was no difference in live birth and pregnancy rates between controlled ovarian stimulation (COS)-IUI and IUI.13,14 Recently, sperm DNA fragmentation has also been taken into account while managing male sub-fertility, with it stated that a higher (>30%) DNA fragmentation index may lead to poor chances of success in an IUI cycle. However, there is insufficient evidence (Level C) to recommend the use of DNA integrity tests to predict pregnancy with IUI.15
Endometriosis
Level II evidence suggests that COS with IUI is a viable treatment option in women with American Fertility Society (AFS)/American Society for Reproductive Medicine (ASRM) Stage I and II endometriosis after laparoscopic correction of the disease.16 Although tubes are patent in endometriosis, adhesions and altered pelvic anatomy do not give encouraging results in IUI. In women with minimal to mild endometriosis, IUI with controlled ovarian stimulation may be effective in increasing live birth rate (OR: 5.6; 95% CI: 1.18–17.4) compared with expectant management. Furthermore, IUI with COS may be more effective at increasing pregnancy rates (OR: 5.1; 95% CI: 1.1–22.5) than IUI alone and may be as effective in women within 6 months of surgical treatment as in women with unexplained infertility.17 IVF is a better choice in Stage III and IV endometriosis, after 2–3 failed IUI cycles in early stage disease, or if associated factors are present, such as diminished ovarian reserve, advanced female age, compromised tubal function, or poor semen parameters.
Anovulatory infertility
In principle, IUI is not an indication in anovulatory infertility, yet most ovulation induction cycles are combined with IUI to obtain better results. IUI can be considered in women with polycystic ovarian syndrome if there is an associated male factor or in women who failed to conceive despite successful induction of ovulation. The clinical pregnancy rates per cycle ranged from 11–20% and the multiple pregnancy rates ranged from 11–36%. Current recommendations suggest IUI should not be first-line therapy in anovulatory women.18
Cervical factor subfertility
Abnormalities of cervical-mucus production or sperm-mucus interaction might impair fertility. The diagnosis is established by a negative post-coital test despite normal semen parameters and coital function, although nowadays the routine use of post-coital test is not recommended while evaluating an infertile female.11,19 Though a higher pregnancy rate was found following IUI in comparison to expectant management (51% versus 33%) in couples with isolated cervical factor,20 IUI was found to be an ineffective treatment for cervical hostility in a systematic review of five trials.21
Tubal factor infertility
Laparoscopic assessment or IVF is suggested for women with bilateral tubal disease or distal tubal occlusion on hysteron-salpingography. COS-IUI can be suggested as initial treatment in isolated unilateral proximal tubal occlusion. However, the cumulative pregnancy rate after three cycles was lower in couples with unilateral tubal disease in comparison to couples with unexplained infertility (30.9% versus 42.6%).22
Number of intrauterine insemination cycles
There is a general consensus in the literature that between four and six IUI cycles may be performed in appropriately selected patients with reasonable pregnancy rates before resorting to alternative treatments. In a retrospective analysis of 15,303 IUI cycles, it was found that the ongoing pregnancy rate (OPR)/cycle decreased from 7.4% in the first cycle to 4.7% and 4.6% in the sixth and ninth cycle, respectively, but the cumulative OPR was 18.3%, 30.3%, and 41.2% after three, six, and nine cycles, respectively.23 We recommend careful evaluation of the patient’s age, duration of infertility, ovarian reserve, pelvic factors, and semen parameters before continuing with IUI treatment beyond three to four cycles.
Ovarian stimulation protocols
Apart from male factors and physical or psychosexual dysfunction where natural cycle IUI can be offered, COS is an integral part of IUI. The rationale of ovarian stimulation is to increase the number of fertilisable oocytes to increase the chances of conception. CC, letrozole, tamoxifen, gonadotropins (human menopausal gonadotropin urinary highly purified FSH, recombinant FSH), combination protocol, and gonadotropin-releasing hormone (GnRH) analogues are used for COS-IUI. In the largest review of 43 trials, involving 3,957 women, the following conclusions were made:24
- Significantly higher pregnancy rates with gonadotropins (OR: 1.8; 95% CI: 1.2–2.7) in comparison to anti-oestrogens.
- No significant difference between anti-oestrogens and aromatase inhibitors (OR: 1.2; 95% CI: 0.64–2.1).
- No significant difference between different types of gonadotropins.
- Gonadotropins alone are more effective than with the addition of a GnRH agonist (OR: 1.8; 95% CI: 1.1–3.0).
- No benefit of adding a GnRH antagonist to gonadotropins (OR: 1.5; 95% CI: 0.83–2.8).
- No evidence of the benefit in doubling the dose of gonadotropins (OR: 1.2; 95% CI: 0.67–1.9), although the multiple pregnancy rates and ovarian hyperstimulation rates were increased.
There are numerous studies demonstrating the effectiveness of one protocol over another; at present, we would recommend a cost effective individualised stimulation protocol for IUI cycles. The approach of mild ovarian stimulation with the aim of two to three follicles with close ultrasound monitoring of the cycle and strict cancellation criteria is optimal.
Mono-follicular versus multi-follicular cycles
Multi-follicular growth is associated with higher pregnancy rates in COS-IUI. In a meta-analysis, the chance of pregnancy was 5% higher with two follicles and 8% higher with three or four follicles. At the same time, the risk of multiple pregnancy was increased by 6%, 10%, and 14% with two, three, and four follicles, respectively.25 In our previous study, we found a cumulative pregnancy rate (CPR) of 19.7% in mono-follicular cycles and 29.4% in multi-follicular cycles.26 Hence, we strongly recommend mono-follicular or bi-follicular cycles, and cancellation should be considered if there are >3 follicles of ≥16 mm on the day of human chorionic gonadotropin (HCG) administration.
Endometrial thickness
Optimal endometrial thickness is essential for conception. There are scarce data regarding the influence of endometrial thickness and pattern on the success of IUI cycles.27 Still, a trilaminar endometrium with thickness ≥8 mm is considered favourable and a thin (<6 mm) or hyperechoic endometrium on the day of HCG administration is not suitable for successful IUI outcome.
Endometrial injury
Intentional endometrial injury is currently being proposed as a technique to improve the probability of pregnancy in women undergoing IVF; however, the effectiveness of this procedure in women attempting to conceive via IUI, expectant management, or TI remains unclear. In a systematic review of nine trials, there was low-quality evidence that endometrial injury may improve clinical pregnancy rates (relative risk [RR]: 1.98; 95% CI: 1.51–2.58). Further high-quality trials are required to confirm these findings.28
Timing of intrauterine insemination
To achieve optimal success, IUI should be done as close to ovulation as possible; hence, the timing of IUI in relation to ovulation is very important.
Ovulatory trigger
According to the analysis of the natural cycle by the World Health Organization (WHO), ovulation occurs 24–56 hours after onset of luteinising hormone (LH) surge (mean time: 32 hours), and after HCG injection ovulation starts 32–38 hours later and is sequential thereafter.29 A mature oocyte is fertilisable for 12–24 hours after release.30 There are multiple methods for synchronisation, like detection of LH surge in urine or blood, administration of urinary (5,000–10,000 international units intramusculatory) or recombinant HCG (250 mcg subcutaneously) or GnRH agonist (leuprolide 1 mg/decapeptyl 0.1 mg/buserelin 0.5 mg subcutaneously), or a combination of them. According to a Cochrane review31 of 14 randomised control trials (RCT) there was no difference in live birth rate (LBR) between HCG versus LH surge or urinary HCG versus recombinant HCG, and HCG versus GnRH agonist. Also, there was no optimal time interval from HCG injection to IUI.31 Documentation of ovulation on ultrasound before IUI is also reported to yield higher pregnancy rates (23.5% versus 8.8%).32
Timing of semen collection, preparation, and insemination
Semen collection for IUI should preferably be done at the clinic. There should be no delay from collection to processing of semen, because prolonged exposure of sperm to seminal plasma results in a marked decline in both motility and viability, hence they must be separated as soon as possible after ejaculation. After semen washing, there are certain changes in the acrosome of the sperm, which initiates capacitation and a progressive decrease in sperm motility due to exhaustion of energy sources.33 Hence, a prolonged sperm wash to IUI interval may lead to a reduced number of fertilisable sperms reaching the fallopian tubes.34 We recommend semen processing within 30 minutes of ejaculation and IUI within 1–2 hours of semen processing to get the best results in terms of clinical pregnancy.
Semen parameters
Volume of inseminate varied between 0.2–1.0 mL in most of the studies. Inseminate volume <0.2 mL would not be sufficient for IUI as it may not compensate for the dead space of an IUI catheter. Similarly, higher volumes of inseminate increases the chances of back-spill and there is no evidence that increasing inseminate volume can increase the chances of conception; hence, the optimal volume of inseminate should be between 0.3 mL and 0.5 mL.
There is wide variation in the literature regarding semen quality and IUI success rates, with a huge difference in the values of various semen parameters. Ombelet et al.35 suggested that the following cut-off values can be taken while considering IUI:
- Inseminating motile count: >1 million.
- Sperm morphology using strict criteria: >4% normal morphology.
- Total motile sperm count in native sample: 5–10 million.
- Total motility in native sample: >30%.
While using these cut-offs, the sensitivity (ability to predict pregnancy) was limited but the specificity (ability to predict failure to conceive) was much better.
Semen preparation techniques
Insemination with unprocessed semen is associated with pelvic infection and it is necessary to remove seminal plasma to avoid prostaglandin-induced uterine contractions. The rationale behind semen preparation in IUI is the separation of motile, morphologically normal, spermatozoa from infectious agents, antigenic proteins, dead sperm, leukocytes, and immature germ cells, which may lead to the production of free oxygen radicals. The most popular methods are simple wash and centrifugation, double density gradient, or swim up. In a systematic review of five RCT, there was insufficient evidence to recommend any specific method of semen preparation.36 The choice of semen preparation method should be decided on the basis of quality of the native sample. The time of centrifugation is more important than the g-force for inducing formation of reactive oxygen species, which may lead to sperm membrane injury; hence, the time of centrifugation should be kept at minimum.37 There are also certain advanced methods for sperm preparation, like hyaluronic-mediated sperm selection, filtration method, and magnetic-activated cell sorting, which are reported to give a higher fraction of motile, viable, and non-apoptotic sperm with high DNA integrity and better cryo-survival rates, but their use in IUI is limited.
Number of inseminations
Insemination is timed around ovulation and can be done once or twice. There is controversy in the literature regarding the number of inseminations for male factor and non-male factor infertility. The rationale of double IUI in a multi-follicular cycle and in male factor subfertility is to widen the fertilisation window so as to deliver more sperm for fertilisation of multiple oocytes released sequentially. In most of the published studies, a single well-timed insemination was done between 34 and 36 hours post HCG administration. Some authors found a benefit of conducting two inseminations, where one was preovulation (around 12–24 hours post-HCG) and the second insemination was conducted post ovulation (between 34 and 48 hours post-HCG).38,39 However, in a meta-analysis by Polyzos et al.40 there was no benefit of double IUI in terms of clinical pregnancy rate in couples with unexplained infertility. In a separate meta-analysis by Zavos et al.,41 there was a trend towards higher pregnancy rates (OR: 2.0; 95% CI: 1.07–3.75; p<0.03) in male factor infertility. In a retrospective study, there was no difference in clinical pregnancy rates in donor insemination with double IUI (single 16.4% versus double 13.6%).42 Therefore, we do not recommend double IUI, as it increases the cost and psychological burden to the couple without increasing the pregnancy rates.
Easy versus difficult intrauterine insemination
Use of tenaculum, uterine sound, or touching the fundus while doing an IUI, back-spillage of semen, blood on the tip of an IUI cannula, and abdominal cramps or spotting post-IUI indicates a difficult IUI. In all these cases, the chances of conception are lower compared to an easy IUI. Studies on IUI under ultrasound guidance are also inconclusive.43,44
Type of catheter
Various types of catheters are available commercially, such as soft or rigid, disposable or metal, with or without stylet. A Cochrane review of nine trials suggested that there was no evidence of significant difference in CPR or LBR with the choice of catheter type.45 We recommend an IUI catheter should be atraumatic, non-toxic, and easy to use.
Abstinence
In natural intercourse, cervical crypts act as a sperm reservoir, providing a supply of sperms for up to 72 hours, but in IUI there is no such reservoir. The fertilisable life span of washed sperms is only 2–3 hours, while it is 12–24 hours for a mature oocyte. Hence, we recommend sexual intercourse around the time of IUI in couples with non-male factor infertility.
Immobilisation post-intrauterine insemination
Sperm may reach the fallopian tubes within 5 minutes of insemination.46 The rationale of immobilisation after IUI is to prevent any leakage of semen and to compensate for the absence of sperm reservoir in cervical mucus that forms after natural intercourse. Significant improvements in cumulative OPR (27% versus 18%) and LBR (27% versus 18%) were reported after 15 minutes of immobilisation versus immediate mobilisation post IUI.47
Luteal phase support
Progesterone is essential for the establishment and maintenance of pregnancy. There is no need for luteal phase support in natural cycle IUI. In cycles stimulated with CC, letrozole, or combined protocols of CC or letrozole with gonadotropins, there is sufficient endogenous LH level that stimulates development of the corpus luteum. On the other hand, in gonadotropin stimulated IUI cycles, supraphysiologic oestradiol levels lead to negative feedback at the hypothalamus, which leads to lower luteal LH levels and therefore to defective implantation. In an updated systematic review and meta-analysis of 11 trials with 2,842 patients, the CPR (RR: 1.56; 95% CI: 1.21–2.02) and LBR (RR: 1.77; 95% CI: 1.30–2.42) were significantly higher with vaginal progesterone supplementation in patients undergoing gonadotropin IUI cycles. The number of treated patients needed to have one additional live birth totals 11.48 In view of the low-cost, ease of administration, and side effects, we recommend the use of vaginal natural micronised progesterone over HCG or dydrogesterone or progesterone gel in gonadotropin-stimulated IUI cycles. At present, there is insufficient evidence regarding use of GnRH agonists at the time of implantation in IUI cycles.49
Complications
Although IUI is a simple minimally invasive procedure, it is not without complications (Table 3). If done properly, the chances of infection are negligible (0.01–0.20%). The most feared complication of IUI is multiple pregnancy, which is related to COS. Most of the studies in the past have reported very high rates of multiple pregnancy with COS IUI (10–40%).25 However, if stimulation is milder and a strict cancellation policy is adopted, it is comparable to IVF single embryo transfer (7% versus 5%).50 The chances of ectopic pregnancy and spontaneous abortion are similar to other infertility treatments.

Table 3: Complications of intrauterine insemination.
COS: controlled ovarian stimulation; IUI: intrauterine insemination; OHSS: ovarian hyperstimulation syndrome.
WHY DOES INTRAUTERINE INSEMINATION FAIL?
IUI is a simple technique that gives reasonable pregnancy rates in selected sets of patients, though it is not without limitations. The main reasons for failure of IUI treatment are:
- Poor patient selection.
- Improper timing of IUI in relation to ovulation.
- Poor semen preparation technique or triple sperm defects.
- Faulty technique or difficult IUI.
- Subtle tubal dysfunction or peritubal adhesions.
- Poor oocyte quality.
- Non-receptive endometrium.
INTRAUTERINE INSEMINATION VERSUS EXPECTANT MANAGEMENT/TIMED INTERCOURSE/INTRACERVICAL INSEMINATION/FALLOPIAN TUBE SPERM PERFUSION/IN VITRO FERTILISATION
There is insufficient evidence for superiority of IUI over expectant management or TI. IUI with frozen semen is superior to intracervical insemination.51 Fallopian tube sperm perfusion was not found to be better than IUI.52 In a recent RCT of 602 couples, COS-IUI was found to be noninferior to IVF single embryo transfer and IVF in a modified natural cycle in terms of time to achieve pregnancy (8.39, 8.04, and 8.32 months, respectively) and LBR (47%, 52%, and 43%, respectively) and was found to be more cost effective.50 The treatment dropout rates were significantly lower with the IUI group. In another study, the cost per pregnancy resulting in at least one live birth was three times higher with IVF compared to IUI.53
COUNSELLING
Counselling is an integral part of any assisted reproductive technique programme. It should be incorporated in the treatment cycle from the beginning, either by the treating fertility specialist themselves or by a psychological counsellor. As we know, infertility and undergoing fertility treatment is stressful for the couple with lots of expectations from every cycle; therefore, they should be informed about the available options, treatment process, prognosis, and cost analysis. Couples should be given an informed choice and assisted at every step so that there are fewer treatment dropouts.
CONCLUSION
This review highlights the current status of IUI in infertility management. IUI should be considered as a first-line treatment option in the management of infertility, considering its simple, patient friendly, non-invasive nature, and cost effectiveness over IVF. The approach of mild ovarian stimulation while minimising the risk of multiple pregnancy, proper timing to enhance success, and with thorough counselling, should be adopted.








