Abstract
Aims and Objectives: To establish the level of serum progesterone (P4) on the day of oocyte retrieval beyond which it can affect the outcome of in vitro fertilisation (IVF), and to further establish the incidence of serum P4 rise in an agonist and antagonist cycle.
Methods: This prospective observational cohort study was conducted from November 2020 to November 2021 at the Sarvodaya Fertility and IVF Centre, Delhi, India. For this study, the author recruited 352 couples with infertility who were treated with IVF/intracytoplasmic sperm injection-embryo transfer, of which 279 patients completed an IVF/intracytoplasmic sperm injection-embryo transfer cycle during the study period and were included in the final analysis.
The standard gonadotropin-releasing hormone antagonist (fixed or variable) and long gonadotropin-releasing hormone agonist controlled ovarian stimulation protocols were used in all patients. Participants were recruited if they were undergoing controlled ovarian stimulation with all gonadotropins, recombinant follicle-stimulating hormone/urinary human menopausal gonadotropin, or recombinant luteinising hormone. The study population was sub-grouped into two groups according to their P4 level on day of oocyte retrieval (calculated according to receiver operating characteristics curve): Group A (p≤11.6 ng/dL; n=247 out of 27; 88.5%) and Group B (p>11.6 ng/dL; n=32 out of 279; 11.5%). Statistical analysis was performed with the Statistical Package for Social Sciences (SPSS) 17.0 version (IBM, New York City, New York, USA).
Results: The percentage of patients with a rise in P4 on the day of oocyte retrieval were found significantly more in the antagonist protocol (13.3% [24 out of 181]) than in the agonist protocol (8.2% [8 out of 98]; p=0.04). Pregnancy rate was significantly higher in Group A (39.3% [97 out of 247]) compared with Group B (12.5% [4 out of 32]). The clinical pregnancy rate was also significantly higher in Group A (34.4% [85 out of 247]) compared with Group B (6.3% [2 out of 32]).
Conclusion: Patients with higher levels of P4 (>11.6 ng/mL) were associated with lower pregnancy and clinical pregnancy rates.
Key Points
1. In this prospective observation cohort study, the author recruited 352 couples with infertility, who were treated with in vitro fertilisation/intracytoplasmic sperm injection-embryo transfer, to determine if the level of serum progesterone (P4) on the day of oocyte retrieval could affect the outcome of in vitro fertilisation treatment.2. An increase in P4, along with high oestradiol levels, could lead to endometrial glandular stromal asynchrony and implantation failure.
3. Identifying the threshold where P4 affects pregnancy outcomes is important as it could aid a practitioner in counselling their patient.
INTRODUCTION
Despite the use of gonadotropin-releasing hormone (GnRH) analogues, subtle increases in serum progesterone (P4) levels beyond an arbitrarily defined threshold value have been observed at the end of the follicular phase in controlled ovarian stimulation (COS) cycles for in vitro fertilisation (IVF). Although the frequency of elevated serum P4 level varies in COS cycles, incidences as high as 35% (5–35%) have been reported in individuals treated with GnRH agonists and 38% (9–38%) in those treated with GnRH antagonists.1-6
In natural cycles, there is a small physiological rise in serum P4 levels prior to ovulation and it is thought to be essential for bringing about an increased luteinising hormone (LH) receptor induction on granulosa cells for enhanced LH action. This rise in serum P4 levels is due to the increased responsiveness of granulosa cells to LH. However, the rise seen in stimulated cycles is far more than that in natural cycles, possibly due to higher number of follicles (stimulated by maintained high follicle-stimulating hormone [FSH] concentrations as a result of daily FSH injections). These follicles will produce and secrete more P4 into the ovarian vein than a single follicle in the normal mid-follicular phase, but with declining FSH concentrations.
In the event of ovarian stimulation-induced multiple-follicle growth, the P4 output to the periphery will be magnified in accordance with the number of follicles and the FSH drive. This is likely to have an impact on the concentration of P4 in the periphery and may influence endometrial development. Thus, the three major components to the degree of P4 secretion from the ovaries will be the number of follicles (or granulosa cells); the degree of trophic stimulus (FSH drive to granulosa cells); and the degree of LH drive to theca cells, which encourages conversion of P4 precursors to androgens and then oestrogen.6
The rise in serum P4 levels then coupled with high oestradiol levels may result in endometrial glandular stromal asynchrony, which may be associated with implantation failure due to a luteal phase defect, while having no influence on oocyte or embryo development.7-10
A large number of clinical studies have examined the effect of a rise in serum P4 levels on the day of human chorionic gonadotropin (hCG) administration in GnRH agonist and antagonist cycles on pregnancy rates. The results of these studies are variable. Possible explanations for the discrepancies in the findings are the use of retrospective study design, use of different protocols of controlled ovarian stimulation, and different cut-off levels for P4 at the time of data analysis. Some studies imprecisely define references for elevated serum P4 levels. Furthermore, there is variation in the statistical methods used to estimate specific circulating P4 limit values and in the precision of P4 measurements that use different immunoassays.11 Most investigators have agreed on a cumulative deleterious effect on pregnancy rates as a result of this supra-physiological rise in P4 in the late follicular phase.12-20
There are a few studies that have showed the association of the rise of P4 on day of oocyte retrieval (OCR) and IVF outcome. Nayak et al.21 showed that implantation rate and pregnancy rate were significantly higher when serum P4 level is <12 ng/dL on the day of OCR in the antagonist cycle.
The day of OCR is a provocative time point, particularly in antagonist cycles as the agent used to prevent the LH surge is stopped 36 hours before OCR. However, the average half-life of the GnRH antagonist (GnRHant), when given in multiple doses, is approximately 20 hours, which ultimately leads to a rapid recovery from pituitary suppression. As a result of this, an endogenous LH surge may occur due to rapid recovery from pituitary suppression, along with an hCG trigger that may raise P4 levels proximally to the retrieval and the result may have a deleterious effect on the endometrial lining.21 Despite this challenging possibility, there have been few studies that have sought to determine the normal distribution of P4 levels at 36 hours after an hCG trigger, or threshold P4 levels at this time point beyond which pregnancy is unlikely.21-23
AIMS AND OBJECTIVES
In this report, the author presents a prospective non-interventional, observational single centre cohort study, which aims to evaluate the association between serum P4 levels on day of OCR and pregnancy outcome in patients undergoing IVF with COS. This study also aimed to establish the incidence of serum P4 rise in agonist and antagonist cycles, as well as in cycles that use drugs such as recombinant FSH (rFSH) and rFSH versus human menopausal gonadotropin cycles.
MATERIAL AND METHODS
This prospectively observational cohort study was conducted from November 2020 to November 2021 at the Sarvodaya Fertility and IVF centre, Delhi, India. There were 352 couples with infertility who were treated by IVF intracytoplasmic sperm injection-embryo transfer (ICSI-ET) who met the inclusion criteria and were included in the study. Participants undergoing COS with all gonadotropins, rFSH/urinary hMG, or recombinant LH were recruited.
P4 levels were obtained at three time points: on Day 2 or 3 of IVF cycles; Day 6 of stimulation in long agonist as well as multiple dose antagonist protocols; and in the morning of OCR. The standard GnRHant (fixed or variable) and long GnRH agonist COS protocols were used for all patients.
Written informed consent was taken from all couples before recruiting the patients in the study.
Inclusion Criteria
All females registered for IVF or IVF/ICSI-ET using an agonist or antagonist protocol were included in this study.
Exclusion Criteria
Individuals were excluded if they had a P4 level >2 ng/mL; ≥2 previous failed IVF cycles; unclipped hydrosalpinx, intramural fibroid ≥4 cm, or localised (>4 cm) or diffuse adenomyosis; donor recipient cycles; were aged more than 37-years-old; or were on dehydroepiandrosterone acetate at the start of the stimulation.
Cancellation Criteria
Some patients were unable to continue in the study as a result of poor response (≤3 oocytes retrieved); endometrium being ≤6 mm on the day of OCR; having embryos with poor morphology on Days 2, 3, or 5; and the embryo transfer not being completed.
Outcome Measures
The primary outcome measure was the pregnancy rate in females undergoing IVF. However, the author also looked at several secondary outcome measures, including clinical pregnancy rate; pregnancy loss rate; ectopic pregnancy rate; incidence of serum P4 rise in various protocols; and incidence of serum P4 rise with cycles using drugs such as rFSH/LH versus only rFSH and rFSH versus hMG cycles.
The pregnancy rate included looking at the number of patients with serum β-hCG >20 IU/mL on Day 14 after OCR, divided by the total number of cycles. Meanwhile, the clinical pregnancy rate is the number of clinical pregnancies expressed per 100 completed cycles.
In this study pregnancy loss meant a miscarriage at 12 weeks. An ectopic pregnancy is where implantation takes place outside the uterine cavity.24
Hormonal Assay
Blood samples were drawn at three designated time points. The blood was collected aseptically and allowed to clot as soon as possible. No additives or preservatives were required to maintain integrity of the sample. The sample was analysed for P4 with the LIAISON® progesterone assay (DiaSorin, Saluggia, Italy), which is a chemiluminescent immunoassay to be used with the LIAISON analyser (DiaSorin) for quantitative determination of P4 in human serum.
STATISTICAL ANALYSIS
Sample Size Calculation
At the Sarvodaya Fertility and IVF Centre, the pregnancy rate in patients with infertility undergoing IVF ranges from 35–40%. For the sample size calculation, the author expected a pregnancy rate of 35%, with a precision error of estimation of 6% and an alpha of 0.05; therefore, a sample size of at least 245 cases was needed. However, the author had taken at least 300 cases to counteract any cases that dropped out. The sample size was calculated using this formula: (Z²×p×q)/d².
Statistical Methods
Statistical testing was conducted with the Statistical Package for Social Sciences (SPSS) 17.0 version (IBM, New York City, New York, USA). Continuous variables are presented as mean±standard deviation, while categorical variables are presented as absolute numbers and percentage. Data were checked for normality before statistical analysis. Normally distributed continuous variables were compared using the unpaired student’s t test, whereas the Mann–Whitney U test was used for those variables that were not normally distributed. Categorical variables were analysed using either the chi-square test or Fisher’s exact test. A receiver operating characteristics (ROC) analysis was calculated to determine the optimal cut-off values for (mention those variables name). The area under the curve and its 95% confidence interval. For all statistical tests, a p value of less than 0.05 was taken to indicate a significant difference.
RESULTS
In the author’s study, 352 patients were recruited. During the study period, 279 patients completed an IVF/ICSI-ET cycle and were included in the final analysis. Seventy-three patients did not meet the final inclusion criteria because they either had poor response (n=11); their endometrial thickness on the day of OCR was ≤6 mm (n=4); the embryos were poor morphology (n=14); or they did not undergo immediate embryo transfer (ET) because of suspected ovarian hyperstimulation syndrome (n=42). Two patients did not undergo ET due to having a fever as a result of an Upper respiratory tract infection on the day of transfer. The protocols used in patients were either agonist (n=98) or antagonist (n=181). Forty-two patients out of 279 took LH in addition to rFSH for gonadotropin stimulation and 12 patients took only hMG for gonadotropin stimulation.
Only the patients with P4 levels of <2 ng/dL on Days 2 or 3 of the treatment cycles were recruited in the study. P4 levels on Day 6 of cycle were not associated with pregnancy outcome.
Using a ROC curve (Figure 1) on the day of OCR, the area under the curve was 0.537, with a 95% confidence interval. The serum P4 level above this it affects IVF outcome: 11.6 ng/dL.
The study population was sub-grouped into two groups according to P4 level on the day of OCR (calculated according to the ROC curve): Group A (p≤11.6 ng/dL; n=247 out of 279; 88.5%) and Group B (p>11.6 ng/dL; n=32 out of 279; 11.5%).
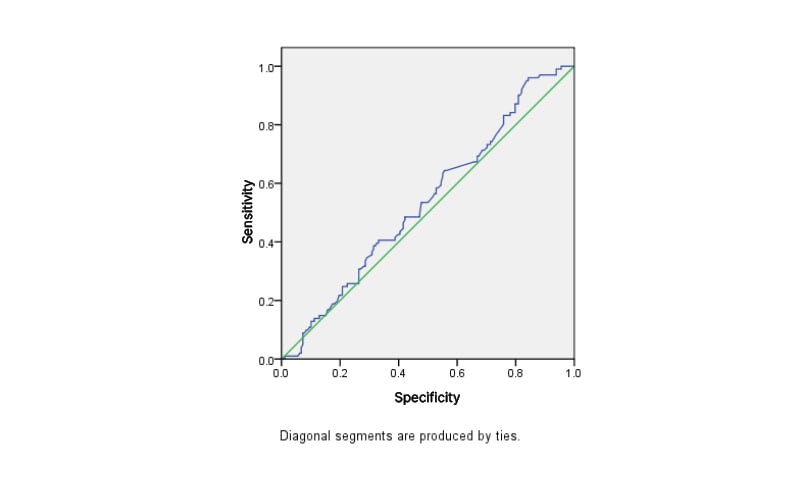
Figure 1: Receiver operating characteristic curve on the day of oocyte retrieval.
The diagonal segments are produced by ties.
The demographic variables shown in Table 1 were similar in two groups.
The percentage of subjects with P4 rise on the day of OCR were found significantly more in antagonist protocol (13.3% [24 out of 181]) than in agonist protocol (8.2% [8/98]; p=0.04). There was no association found in the rise of P4 with using rFSH alone versus the addition of recombinant LH (p=1.000) for superovulation and also in recombinant gonadotropins versus urinary hMG (p=0.635; Table 2).
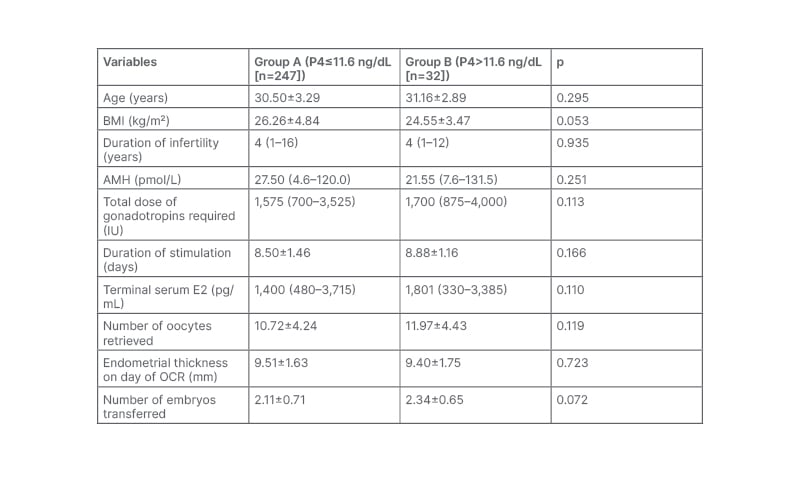
Table 1: The demographic variables of patients Groups A and B. Data is presented as mean±SD or median as applicable.
AMH: anti-Müllerian hormone; E2: oestradiol; OCR: oocyte retrieval; SD: standard deviation; P4: progesterone
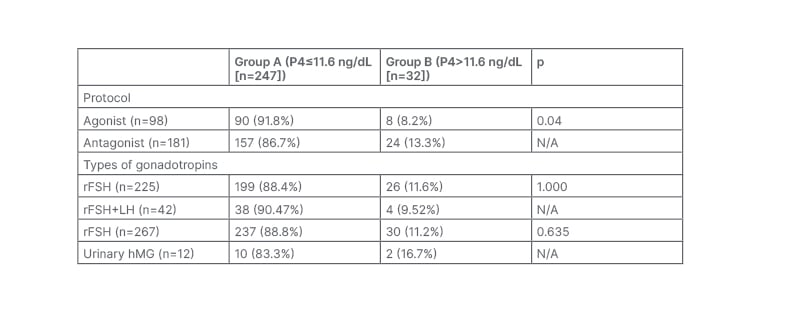
Table 2: The differences between Groups A and B depending on the protocol and type of gonadotropin used.
hMG: human menopausal gonadotropin; LH: luteinising hormone; rFSH: recombinant follicle-stimulating hormone.
Pregnancy rate was significantly higher in Group A (39.3% [97 out of 247]) when compared with Group B (12.5% [4 out of 32]). The clinical pregnancy rate was also significantly higher in Group A (34.4% [85 out of 247]) when compared with Group B (6.3% [2 out of 32]). Early pregnancy loss was not significantly different in both groups (p=0.728) and the incidence of ectopic pregnancy were similar in both groups (p=0.217; Table 3).
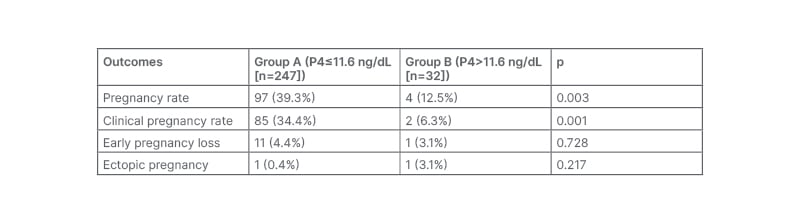
Table 3: The outcomes of the study.
P4: progesterone.
DISCUSSION
This prospective observational study was performed with 279 IVF/ICSI-ET cycles to establish the level of serum P4 on day of OCR to determine how it can affect the outcome of IVF and to further establish the incidence of serum P4 rise in an agonist and antagonist cycle, as well as in cycles using drugs such as rFSH/LH versus only recombinant FSH and rFSH versus hMG cycles. Using the ROC curve, a serum P4 level of 11.6 ng/dL on day of OCR was identified as the most appropriate threshold to define detrimental levels of serum P4 for the outcome of IVF/ICSI-ET cycles.
It showed that P4 rise (>11.6 ng/dL; n=32) on the day of OCR was significantly more in the antagonist group (13.3% [24 out of 181]) compared with the agonist group (8.2% [8 out of 98]). This result is in consistent with the Nayak et al.21 study, which evaluated the distribution of p levels on the day of OCR as it relates to the outcome of pregnancy in an antagonist protocol, which may be at higher risk for elevated p levels. The day of OCR is a provocative time point, particularly in antagonist cycles, as the GnRHant used to prevent the LH surge is stopped 36 hours before OCR. However, the average half-life of GnRHant (when given in multiple doses) is approximately 20 hours, which ultimately leads to a rapid recovery from pituitary suppression. As a result of this, an endogenous LH surge may occur due to rapid recovery from pituitary suppression, along with hCG trigger that may raise P4 levels to be more proximal to the retrieval and the result may have a deleterious effect on the endometrial lining.21
The primary reason why there were 91 patients treated with the agonist protocol and 181 patients were treated with the antagonist protocol was because, at the author’s centre, the antagonist protocol is the most commonly used in patients. The patients using agonist protocol were included as none of the studies establish the incidence of serum P4 rise in an agonist and antagonist cycle. This is the only study that shows that P4 rise (>11.6 ng/dL; n=32) on the day of OCR was significantly higher in the antagonist group (13.3% [24 out of 181]) compared with the agonist group (8.2% [8 out of 98]).
This study also showed that P4 rise (>11.6 ng/dL; n=32) on the day of OCR was associated with a significantly decreased pregnancy rate and decreased clinical pregnancy rate after fresh embryo transfer in females undergoing ovarian stimulation for IVF. The pregnancy rate observed was significantly higher in normal P4 level (≤1.6 ng/mL; n=247) group than in elevated P4 level (>11.6 ng/mL; n=32) group (39.3% [97 out of 247] versus 12.5% [4 out of 32]; p=0.003). The clinical pregnancy rate observed was significantly higher in normal P4 level (≤11.6 ng/mL; n=247) group than in elevated P4 level (>11.6 ng/mL; n=32) group (34.4% [85 out of 247] versus 6.3% [2 out of32]; p=0.001).
A study by Niu et al.22 examined P4 levels on the day of OCR in cycles with GnRH agonists (long and short protocols) and found that P4 levels correlated with the number of oocytes and embryos but could not predict the outcome of the pregnancy. In a prospective study of 186 females, Nayak et al.21 examined the prediction of assisted reproductive technology success depending on P4 level on the day of OCR in patients on a short stimulation protocol with GnRHant. Patients with a P4 rise (>12 ng/mL) on the day of OCR had lower pregnancy and implantation rates in this study.20 Fernandez et al.23 performed a prospective cohort study of 400 IVF/ICSI cycles, with a fresh embryo transfer on day 2or 3. They proposed a serum P4 level on percentile 90 as a threshold. Pregnancy rates were not affected; however, there were more miscarriages (25.7% versus 43.8%) and a lower live birth rate (28.0% versus 23.1%) in the P4 rise group, which is not statistically significant.23 There was no statistically significant difference found in demographic variables and use of type of gonadotropins in this study.
The advantage of identifying a P4 threshold beyond which pregnancy outcomes may be affected can aid in the practitioner’s ability to counsel a patient. Using time more proximally to OCR, the author discovered that a P4 level of >11.6 ng/mL may be predictive of significantly poorer pregnancy and clinical pregnancy rates.
These results have several important clinical considerations. Firstly, the negative effect of an elevated P4 at the time of OCR appears to be limited to the endometrium, as no effect on oocyte maturation or fertilisation rate was detected; this has been corroborated by previous studies in donor recipient IVF cycles as well as in frozen embryo cycles.7-10 Therefore, a simple solution may be cryopreserving embryos when P4 levels exceed this threshold. Thus, the P4 level on the day of OCR may help to stratify which patients may benefit from embryo cryopreservation in lieu of a fresh ET. Secondly, this article hypothesises that there may be a benefit in continuing the GnRHant beyond the day of OCR, not only prevent the rise in P4 levels but to also prevent the potential cumulative negative effect that the premature rise may have on the endometrium after an hCG trigger. As the cumulative negative effect on the endometrium may be abated in this fashion and thus lead to an improvement in pregnancy rates; however, this has not yet been studied, and any potential adverse effects of the antagonist on the embryo would need to be considered.
CONCLUSION
This study found a significant correlation between the levels of P4 on the day of OCR and a positive outcome for assisted reproductive technology procedure. Patients with lower levels of P4 (≤11.6 ng/mL) were more likely to achieve pregnancy and deliver a healthy baby. In patients contemplating ET in favour of subsequent frozen ET, P4 levels at OCR may be one more piece of information that would impact their decision.








