Meeting Summary
With individualised treatment becoming an increasingly relevant topic in reproductive medicine, this symposium discussed how new and existing evidence can support a more patient-centric approach to fertility treatment. Co-Chair Prof Filicori opened the symposium by welcoming delegates and taking a moment to reflect on some of the key milestones in fertility treatment over the past few decades, including approaches that are currently being used to facilitate an individualised approach to controlled ovarian stimulation (OS). Prof Baker continued the theme of individualisation by discussing how the use of different data sources, such as randomised controlled trials (RCT), observational studies, and prediction models, could help guide personalised care. Dr Raine-Fenning presented results from the recent MEGASET-HR trial, which compared the efficacy of highly purified human menopausal gonadotrophin (HP-hMG) versus recombinant follicle-stimulating hormone (rFSH)α in patients predicted to be high responders based on their anti-Müllerian hormone (AMH) levels. The results of this study build on the existing evidence for human chorionic gonadotrophin (hCG)-driven luteinising hormone (LH) activity (HP-hMG) and provide exciting and practical insights on tailoring treatment in this subgroup of patients at risk of ovarian hyperstimulation. Dr Wijngaard-Boom then presented new data from the follitropin delta ESTHER clinical trial programme as well as real-world experience from her own clinic in Rotterdam. The real-world data presented showed that individualised follitropin delta dosing based on the approved algorithm delivers a predictable ovarian response, which is consistent with the results from the ESTHER registration trials, thereby offering positive reassurance about the role of follitropin delta in a clinical setting. The symposium was closed by Co-Chair Prof Laven, who concluded that the approaches discussed during the symposium demonstrate how treatment can be individualised based on a patient’s characteristics, and that, if they are not already, fertility experts should be looking to individualise the treatment for each of their own patients.
Treating a Diverse Assisted Reproductive Technologies Population: Applying Statistics to the Individual Patient
Professor Valerie Baker
Infertility specialists encounter a diverse range of patient types with varying characteristics and profiles that can impact on their pregnancy outcomes. Clinicians are increasingly moving towards an individualised approach in order to provide each patient with the greatest chance of success. For instance, this can be performed by tailoring the treatment pathway (mild versus conventional versus aggressive stimulation, fresh embryo transfer versus frozen embryo transfer, banking [oocytes or embryos]), and considering factors such as the underlying cause of infertility and prior treatment (Figure 1).1 Patient goals should also be taken into account, as concerns such as safety, cost, and risk of transmitting a genetic disease may all influence the choice of treatment.
Figure 1. Infertility diagnosis and prior treatment considerations when selecting the optimal treatment pathway for individual patients.
IVF: in vitro fertilisation; OHSS: ovarian hyperstimulation syndrome.
Despite the fact that patients ultimately have the same objective, the variety of treatment options and characteristics means that patient journeys may end up being vastly different. With this high level of heterogeneity, it can be difficult for clinicians to decide which treatment option is most appropriate for their patient. There are several data sources available from which valuable insights are being gained on different treatment approaches, including surveillance data, electronic health records (EHR), RCT, observational data, and predictive models.
Surveillance data has been shown to be a rich data source for clinical outcomes research in reproductive medicine and is relatively inexpensive to obtain.2 Registries are being set up around the world, with databases already established in the USA, Europe, Latin America, and Africa. One of the largest databases is the Society for Assisted Reproductive Technology Clinical Outcomes Reporting System (SART), which captures around 90% of the cycles performed in the USA and records information such as patient demographics, baseline, and treatment parameters, as well as outcomes.3 Due to the large amount of data collected, these databases can be used to assess a diverse, real-world population and can be statistically powered for key outcomes such as live birth. However, as these data are not solely collected for research purposes, this method of data collection currently falls short in terms of the data available on confounding factors, and there is still the potential for incomplete or selective reporting of in vitro fertilisation (IVF) cycles.2
EHR are another source of data used to investigate clinical practice patterns, thereby providing real-world insights.4 The costs associated with EHR are relatively low, as data have already been collected, but there are several limitations associated with this source that relate to the availability of the data. There are still several parameters, including collection of OS data, that are not well captured. Furthermore, many healthcare systems continue to use paper records, at least in part, and the records themselves are not fully integrated across practices.4
The RCT has long been the gold standard for determining efficacy of therapeutic interventions.5 Randomisation can address the issue of bias present in other clinical study designs, such as case-controlled studies and cohort studies, allowing potential confounding factors to be balanced in both treatment arms.5 There are limitations to RCT, such as their high costs, which means that there are only a small number of adequately powered trials conducted with a suitable follow-up period.5,6 The consequences of underpowering RCT are significant and can leave large data gaps.6 In reproductive medicine, even the largest trials have relatively limited power to assess key outcomes.6 A recent analysis suggests that the largest trials in reproductive medicine only had around a 35% power to detect a difference in live birth rate (LBR) of 10 percentage points. This limitation could not be overcome by meta-analyses, which still only have <50% power to detect a difference in LBR of 10 percentage points.6 Another limitation of RCT is that the outcome is normally reported as an average treatment effect, which will not be experienced by most patients.5 Furthermore, the results from RCT are not always applicable to many real-world patients due to the specific inclusion and exclusion criteria applied to the RCT study population.7
Where there is a lack of RCT data, clinical decisions are often based on evidence from observational studies. Clinical trial emulations and transportability analyses can be combined with methods for examining heterogeneity of treatment effects, facilitating a personalised treatment approach. One use of observational studies is to emulate a hypothetical target trial that would address the clinical question of interest.7 For example, by defining a suitable follow-up period and outcomes of interest (e.g. live birth), an observational study could be used to analyse patients, as if randomised, to estimate treatment effect. This provides a method to impose structure on real-world data. Observational studies can also be used in situations where trial evidence is available but not applicable. Statistical and epidemiological models can be applied to observational data and clinical trial data to transport inferences from trial participants to a real-world population.7
Predictive modelling is another valuable tool that can be used to guide treatment recommendations and counsel patients. Using data sources such as observational datasets and clinical trial data, an algorithm can be applied to patient baseline characteristics in order to predict outcomes such as number of oocytes retrieved.1
Through a combination of the sources described above, individualised strategies are increasingly being implemented in everyday clinical care. In particular, methods to predict ovarian response are extensively used in clinical practice, with AMH and antral follicle count (AFC) becoming widely accepted as the most practical and sensitive markers of ovarian response available.8,9 To date, there have only been a small number of RCT prospectively assessing the usefulness of individualised dosing compared with standard dosing.10-14 These studies suggest that while individualised dosing is associated with some advantages with regards to safety and cost, there is no distinct benefit in terms of pregnancy rates.15 Future studies comparing personalised and standardised one-size-fits-all treatment approaches will need to consider both efficacy and the benefit–harm balance for the individual patient.
In conclusion, patients all want their care to be individualised and clinicians want to consider the unique characteristics of each patient to minimise risk and maximise benefit. In order to achieve these goals, care will need to be personalised, and guided by evidence from RCT, observational studies, and prediction models.
Building Upon the Evidence for Human Chorionic Gonadotrophin-driven Luteinising Hormone Bioactivity
Doctor Nick Raine-Fenning
HP-hMG is a well-established product for OS, containing follicle-stimulating hormone (FSH) and hCG. There has been significant investment in a large body of trials conducted over the past few decades that have consistently demonstrated the efficacy of hCG-driven LH bioactivity in OS. Studies including EISG,16 MERiT,17 and MEGASET,18 all of which have compared gonadotrophin treatment in both IVF and intracytoplasmic sperm injection (ICSI) cycles, demonstrated comparable results between HP-hMG and Chinese hamster ovary (CHO) cell-derived rFSHα or rFSHβ with respect to ongoing pregnancy rate (OPR). In addition, a recent meta-analysis comparing HP-hMG with rFSHα or rFSHβ across seven studies, including the three RCT described above, concluded that HP-hMG is associated with a higher LBR.19
The MEnopur in GnRH Antagonist Cycles with Single Embryo Transfer (MEGASET) trial compared HP-hMG and rFSHβ in women undergoing a gonadotrophin-releasing hormone (GnRH) antagonist protocol with single-blastocyst transfer.18 The trial established non-inferiority of HP-hMG, with both groups showing similar results in terms of biochemical pregnancy, clinical pregnancy, ongoing pregnancy, and live birth.
Secondary analysis of the MEGASET trial data investigated the ovarian response and clinical outcome in predicted high responders (defined based on serum AMH levels) treated with either HP-hMG or rFSHβ.20,21 The analysis revealed HP-hMG was associated with a significantly higher LBR compared with rFSHβ among patients with AMH levels in the highest quartile.20 Additionally, HP-hMG was associated with a favourable safety profile among these patients, who had a trend towards a lower incidence of early ovarian hyperstimulation syndrome (OHSS) and/or safety interventions due to excessive ovarian response compared with rFSHβ.21
Although fertility experts strive to eliminate OHSS from their clinics, a recent meta-analysis indicated that OHSS still occurs, despite the increased use of antagonist protocols, agonist triggering, and a lower threshold to convert to a freeze-all protocol.22 The results from the MEGASET secondary analysis suggest that the specific gonadotrophin chosen for treatment may influence the risk of OHSS, and therefore influence clinical outcomes in predicted high responders. However, as the analysis was not powered to specifically investigate the treatment effect in these women, more data were needed to support the choice of gonadotrophin in high responders (Figure 2).
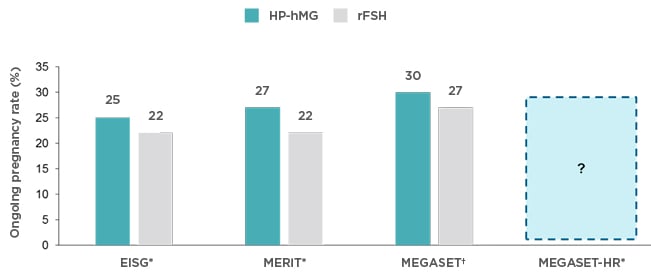
Figure 2. MEGASET-HR is the fourth large randomised controlled trial comparing HP-hMG and rFSH (rFSHα or rFSHβ).
Data provided is per protocol populations.16-18
HP-hMG: highly purified human menopausal gonadotrophin; rFSH: recombinant follicle-stimulating hormone.
*rFSHα; †rFSHβ.
The MEnopur in GnRH Antagonist Cycles with Single Embryo Transfer – High Responder (MEGASET-HR) trial (NCT02554279)23 was specifically designed, therefore, to compare the efficacy and safety of HP-hMG (Menopur®) versus rFSHα (Gonal-f®) in predicted high responders. The study objective was to demonstrate non-inferiority of HP-hMG (Menopur) versus rFSHα (Gonal-f) with respect to OPR in women undergoing OS in a GnRH antagonist protocol. The study was a randomised, assessor-blind, non-inferiority clinical trial carried out at 31 infertility centres across the USA. Patients predicted to be high responders (defined based on serum AMH levels) were enrolled to undergo ICSI treatment using a GnRH antagonist protocol with a fresh, single-blastocyst transfer. A follow-on study looked at frozen cycles, thereby allowing assessment of cumulative pregnancy rates. The methods and results of this trial were presented during the symposium and will be reported in the full primary publication.
MEGASET-HR will be the fourth in the series of large RCT comparing HP-hMG with rFSH (rFSHα or rFSHβ).16-18 The investigators hope that the outcomes of MEGASET-HR will build on the existing evidence for the safety and efficacy of HP-hMG and provide further insights surrounding the use of gonadotrophins in high responders.
Follitropin Delta: New Evidence and Growing Clinical Experience
Doctor Pauline Wijngaard-Boom
Follitropin delta is uniquely expressed in a cell line of human origin, which produces an individual glycosylation pattern similar to that of naturally derived human FSH.24 Although it has an identical amino acid sequence to CHO-derived rFSH, follitropin delta’s more human glycosylation features determine different pharmacokinetic and pharmacodynamic profiles. As a result, the receptor-binding profile and clearance rate of follitropin delta differs from other forms of rFSH when administered at an equal international unit (IU) dose (as determined by the Steelman–Pohley assay in rats).25 The lack of comparability with existing rFSH preparations means that the Steelman–Pohley assay is not appropriate for measuring follitropin delta activity in humans, and follitropin delta is therefore dosed in micrograms (mcg).25
Due to the distinct properties of follitropin delta and need for improved predictability of response to gonadotrophin dosing, it was necessary to develop an algorithm for individualised dosing. Phase I data were modelled to demonstrate that serum concentrations of rFSH were directly related to the dose and inversely related to body weight.26 As a result, body weight was considered to be a key variable for the development of a follitropin delta dosing algorithm. A dose–response model was also developed to predict the number of oocytes retrieved with follitropin delta.27 The model used several predictors of ovarian response obtained at baseline (age, weight, AFC, serum AMH, serum FSH, and serum inhibin B). Data from the dose–response model indicated that measuring AMH and body weight only are sufficient to predict the ovarian response following follitropin delta treatment.27 Another key step in the development of the algorithm was to determine appropriate dosing for women with different levels of AMH in Phase II studies.27
The follitropin delta dosing algorithm was designed to induce optimal OS to maximise pregnancy, while minimising the risk of OHSS.13 Although follitropin delta is the latest addition to the rFSH treatment landscape, there is already a growing body of clinical evidence to validate the follitropin delta dosing algorithm and support its efficacy across a range of patient types. The Evidence-based Stimulation Trial with Human rFSH in Europe and Rest of World (ESTHER) programme, which consisted of two Phase III trials, was conducted to prospectively validate the follitropin delta dosing algorithm and further support the efficacy and safety profile of follitropin delta.13,28 ESTHER-1 was a randomised, assessor-blind, controlled, non-inferiority, efficacy trial comparing the treatment strategy of individualised follitropin delta dosing with that of conventional follitropin alfa (Gonal-f) for IVF/ICSI.13 ESTHER-2 was a continuation of ESTHER-1, focussed on immunogenicity, covering up to two repeated treatment cycles in women who did not achieve an ongoing pregnancy in ESTHER-1.28 The ESTHER studies used a GnRH antagonist protocol with a single-blastocyst transfer. The coprimary endpoints of the study were OPR (10–11 weeks after transfer) and ongoing implantation rate (predefined non-inferiority margin of –8.0%); the secondary endpoints included distribution of ovarian response, proportion of patients with extreme responses (hypo- and hyper-responses), LBR, early and late OHSS, and/or preventative interventions for early OHSS.13
The dosing of follitropin delta in the first cycle (ESTHER-1) was calculated using the algorithm as follows: in women with AMH ≥15 pmol/L, the daily dose was based on the actual AMH value and body weight; in women with AMH <15 pmol/L, a fixed daily dose of 12 mcg was administered irrespective of body weight.13 The dosing algorithm set the maximum daily dose at 12 mcg in the first treatment cycle to avoid overexposure in patients with higher body weight. Once calculated, the daily dose of follitropin delta was fixed throughout stimulation and was only adjusted in subsequent cycles of OS according to the response of the previous treatment cycle (ESTHER-2).28 Patients receiving follitropin alfa had a starting dose of 150 IU filled-by-mass for the first 5 days, after which the dose could be adjusted according to the ovarian response.13
The main efficacy results from ESTHER-1 were presented during the symposium. The study met its coprimary endpoints of non-inferiority, with similar OPR and ongoing implantation between individualised follitropin delta and conventional follitropin alfa.13 There were also similar results between treatment groups for most of the secondary endpoints, including the number of live births and the number of neonates alive at 4 weeks.13
As a first step towards validating the follitropin delta dosing algorithm, data from a Phase I trial were modelled to predict whether individualised dosing would have an effect on the ovarian response.13 At trial enrolment, the model predicted that 42% of the individualised dosing group would yield an average of 8–14 oocytes. The results from ESTHER-1 validated this prediction, with 43% of patients attaining the predicted target yield.13
Results from the ESTHER clinical trial programme indicated that the follitropin delta dosing algorithm is associated with a favourable safety profile. A recently published secondary analysis of the combined data from ESTHER-1 and ESTHER-2 reported the favourable impact of individualised dosing with follitropin delta as a preventive strategy for OHSS risk in subsequent OS cycles, with advantages observed mainly in patients with the highest AMH levels.28 Unpublished analyses from the ESTHER clinical trial programme were also summarised, including the effect of body weight and AMH levels on oocyte yield, and how these parameters could be used to adjust follitropin delta dosing in order to achieve an appropriate oocyte output for each patient. In addition, a new analysis on how the number of oocytes retrieved affected the proportion of women receiving agonist triggering and fresh transfers was presented. Key results from the ESTHER clinical trial programme were then compared with real-world data collected from the Erasmus University Medical Center (Erasmus MC) in Rotterdam, the Netherlands. These data confirmed that individualised follitropin delta dosing using the approved algorithm delivers a predictable ovarian response in the population treated at Erasmus MC, with results consistent with ESTHER-1 clinical trial data.
In conclusion, it is important to ensure that the patient is always at the centre of any treatment decision. The ESTHER clinical trial programme validated the follitropin delta algorithm and demonstrated that the individualised dosing of follitropin delta provides a predictable ovarian response, with similar pregnancy outcomes but with a more favourable safety profile. Real-world experience and clinical trial evidence suggest that follitropin delta does provide a valuable tool for implementing individualised personalised treatment in a real-world setting.
Conclusion
The symposium was concluded by Co-Chair Prof Laven, who highlighted the importance of achieving individualised care in assisted reproductive technologies. By using approaches such as shared decision making and applying learnings from data sources such as RCT, fertility experts are increasingly able to tailor their treatment approach. Although RCT have long been considered the gold standard, they are typically performed in a select population, meaning that other data sources, such as observational studies and prediction models, will also be needed to guide individualised approaches. Prediction models using patient characteristics, such as AMH level, have already begun to provide a path toward personalised medicine. AMH is the most sensitive biomarker for ovarian response, and has been used to investigate choice of gonadotrophin in those at greatest risk of OHSS. In the MEGASET-HR study, AMH levels were used to select a predicted high-responder population in order to assess how hCG-driven LH bioactivity may influence safety and efficacy of HP-hMG. The results of MEGASET-HR will be reported in the full primary publication. The value of using patient characteristics to predict ovarian response can also been seen with the follitropin delta algorithm, which uses body weight and AMH to individualise the treatment dose. The follitropin delta algorithm, validated in the ESTHER clinical trial programme, delivers a predictable ovarian response in a real-world setting, consistent with clinical trial data. Prof Laven closed the symposium by highlighting the importance of individualising the treatment approach for all patients, not just in those at risk of poor or high response.








