BACKGROUND AND AIMS
The clinical response to severe acute respiratory syndrome coronavirus 2 varies widely among patients, some of whom may progress to severe COVID-19. Frequently, patients are stratified by a single characteristic (e.g., age or use of supplemental oxygen) to determine treatment allocation and response. A more comprehensive approach using a multivariable prediction tool may help identify heterogeneous treatment effects by risk strata and help guide therapeutic interventions.
MATERIALS AND METHODS
The authors assembled a national cohort of veterans hospitalized between March 1, 2020 and February 28, 2022, with real-time PCR-confirmed severe acute respiratory syndrome coronavirus 2 infection. The authors used patient demographics, laboratory, and vital sign data in stepwise logistic regression to create a mortality risk prediction model stratified into five distinct risk categories for 60-day mortality in patients not treated with corticosteroids. They subsequently incorporated patients who were treated with steroids and estimated the differential steroid effect by risk group on 60-day mortality, by using doubly robust linear regression with inverse probability of treatment weights.
RESULTS
The authors identified 43,222 veterans hospitalized at 130 Veterans Affairs Medical Centers (median age: 71; 72% White) with COVID-19, of whom 22,436 (51.9%) received treatment with systemic steroids. The authors’ final risk prediction model included 16 clinical and laboratory variables and had an area under the curve of 0.834 (Figure 1). Risk quintiles for 60-day mortality ranged from <3% (lowest) to >28% (highest). Unadjusted mortality rates in the validation set were 23.0% for patients receiving steroids, and 11.6% for those not receiving steroids. In the authors’ validation cohort, steroid treatment was associated with reduced odds of death in the lowest risk group (odds ratio: 0.65; 95% confidence interval: 0.44–0.93). They also saw a trend towards increased odds of death in the highest risk group (odds ratio: 1.14; 95% confidence interval: 0.78–1.67), corresponding to an absolute risk increase of approximately 8%.
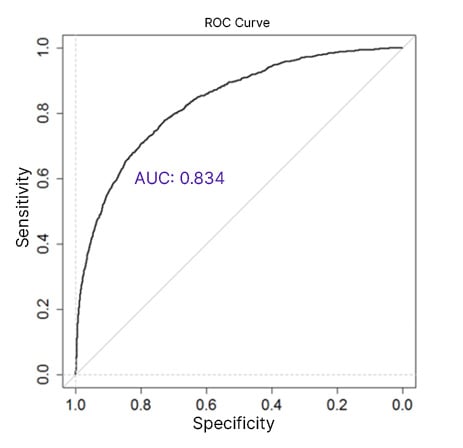
Figure 1: Receiver operative characteristic curve for COVID-19 mortality risk prediction model.
The authors’ mortality risk prediction model incorporated 16 clinical and laboratory variables, and had an AUC of 0.834. The 60-day mortality risk quantiles ranged from <3 to >28%.
AUC: area under the curve; ROC: receiver operative characteristic.
CONCLUSION
In a cohort of elderly multimorbid veterans, the authors observed evidence of decreasing mortality risk benefit due to steroids across groups with increasing baseline risk of death. These results contrast with currently published studies, but may also point to a signal of increased mortality with steroid use seen in a recent large-scale study in the RECOVERY trial group in non-mechanically ventilated patients,1 and an increased rate of ventilator-associated pneumonia noted in patients undergoing mechanical ventilation.2
Future steps will include the identification of and accounting for possibly excluded confounders, including supplemental oxygen use and steroid dosing.








