BACKGROUND AND AIMS
In recent years, there has been a revolution in genomic profiling and molecular typing of lung cancer. EGFR is a key oncogene, and a gold standard for determining EGFR mutation status is a histological specimen taken by a bronchoscopic method or surgical material, but it is often difficult to obtain enough tumour tissue for morphological and molecular verification. Isolation of free circulating peripheral blood tumour DNA (ctDNA), known as ‘liquid biopsy’, is a noninvasive alternative method to tissue biopsy for performing molecular screening in patients suitable for targeted therapy. This study aimed to compare the prevalence of EGFRmutation status in bronchoalveolar lavage (BAL) and peripheral blood according to the gold standard tissue biopsy in patients with primary lung adenocarcinoma.
MATERIALS AND METHODS
All patients scheduled for bronchoscopy in the authors’ hospital between October 2018 and August 2019 were assessed. The exclusion criteria did not differ to those for standard contraindications for a bronchoscopy. Flexible bronchoscopy was performed in all patients under local anaesthesia. The biopsy techniques were forceps biopsy, transbronchial biopsy with or without fluorographic control (based on preliminary CT data), and BAL.
RESULTS
In the period of the study, 140 bronchoscopies were performed. In 112 patients (80%), a tumour was verified. Adenocarcinoma was present in 23.2% (26/112) of the patients with lung cancer, which was verified with immunohistochemistry. The groups did not differ regarding age and tumour maximum diameter. However, there were significantly more females with EGFR mutation (77%; 10/13) as opposed to males (23%; 3/13; p=0.017).
Thirteen patients had wild-type EGFR and the other 13 had EGFR mutation. EGFR mutation from a peripheral blood sample was identified in 38.5% (5/13) of patients, whereas EGFR mutation obtained from BAL was identified in 92.3% (12/13) (Table 1). In one case, in both liquid biopsy samples (BAL and plasma) additional T790M mutation along with deletion in exon 19 were found, which was not established in the corresponding formalin-fixed paraffin-embedded tissue sample (Patient 8). Neither BAL nor blood sample confirmed EGFR mutation in one patient, which was defined as positive only in the tissue sample.
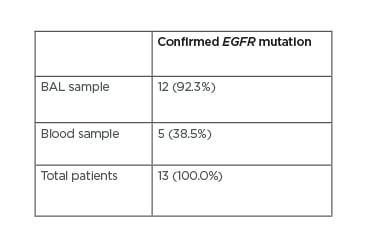
Table 1: Confirmation of EGFR mutation in bronchoalveolar lavage and blood samples.
BAL: bronchoalveolar lavage.
CONCLUSION
The current standard for detection of mutation in patients with unknown status after negative tissue sampling is rebiopsy. Unfortunately, rebiopsy is not always feasible due to many reasons. In this context, liquid biopsy could be an effective solution.
However, results from individual studies are variable,1-3 with many indicating that detection of EGFR mutations in ctDNA is more difficult in plasma samples than in tumour tissue, with an average sensitivity of 65–70%. On the other hand, establishing EGFR status from BAL is a viable and safe procedure, especially in impaired patients with promising results.
In this study, the authors demonstrated the superiority of BAL to venous blood liquid biopsy in establishing an EGFR mutation. This could facilitate a diagnosis with a minimally invasive method and lower risk for the patient with comparable results to formalin-fixed paraffin-embedded tissue.








