BACKGROUND
Bronchopulmonary dysplasia (BPD) and retinopathy of prematurity (ROP) are two severe disorders that arise in the lungs and eyes of preterm infants exposed to prolonged supplemental oxygen in neonatal intensive care units (NICU).1,2 Due to the lack of a clinically relevant animal model that simultaneously replicates the acute and chronic pathology in both the eye and lung, the shared molecular pathways underlying the development of these two disorders remain unexplored.3 To address this gap, the authors utilised the gold standard oxygen-induced retinopathy model, which is widely used to study ROP, to assess coincident development of lung pathology.
METHODS
Neonatal C57BL/6 mice were exposed to supplemental oxygen (75% O2) for 5 days from postnatal (PN) Day 7 to PN12, returned to room air, and assessed at either PN12 or PN18 alongside age-matched room air controls.4 The room air phase was also extended out to PN40 and PN80 to examine late-stage retinal and alveolar lesions (Figure 1). Mouse retinas were whole-mounted and stained with fluorescein isothiocyanate-conjugated isolectin B4 to show acute blood vessel changes at PN12 and PN18, and at PN40 and PN80 eye paraffin sections were stained with haematoxylin and eosin to examine for chronic choroidal pathology. Concurrently, lung paraffin sections from all timepoints were stained with haematoxylin and eosin to examine progressive alterations in alveolar structure of the lungs in the same mice assessed for eye pathology. Bronchoalveolar lavage (BAL) fluid was used to determine the concentration of inflammatory cells in the airspaces, and BAL cell cytology was used to assess cellular activation state.
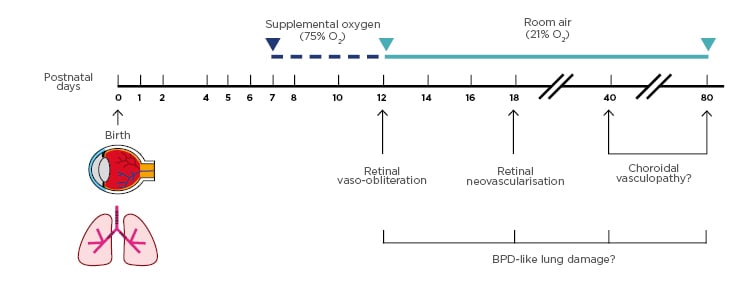
Figure 1: Schematic of the extended gold-standard oxygen-induced retinopathy model used to assess concurrent eye and lung pathology.
At birth, C57BL/6 mouse pups were kept in room air for 7 days. From postnatal (PN) Days 7–12, mice were exposed to supplemental oxygen (75% O2). Mice were either examined at PN12 or returned to room air and studied at PN18, PN40, or PN80. In the retina, vaso-obliteration was detected at PN12, and characteristic retinal neovascularisation was observed at PN18. Choroidal vasculopathy was evaluated at later time-points to assess the long-term impact of the supplemental oxygen insult. To determine if the oxygen-induced retinopathy model replicates the key characteristics of bronchopulmonary dysplasia, the lungs of mice were simultaneously examined at the same four timepoints.
RESULTS
Animals exposed to 75% O2 from PN7–PN12 exhibited BPD-like alveolar wall thickening in the lungs at PN12 and PN18, alongside ROP-like inner vascular degeneration in the retina. At PN40 and PN80, severe airspace enlargement and simplification were observed in lungs of oxygen-exposed mice, with concurrent thinning of the choroid layer in the eye, successfully reproducing the key features of acute and chronic human BPD and ROP. Immune cell infiltration was also elevated in the airspaces of supplemental oxygen exposed mice at PN12 and PN18. Morphologically, alveolar macrophages in the BAL of the supplemental oxygen-exposed mice were enlarged and activated compared to the small, quiescent alveolar macrophages in the airspaces of room air control mice.
CONCLUSION
This study demonstrates that the oxygen-induced retinopathy model not only recapitulates clinically relevant ROP but also successfully yields a pathology reminiscent of BPD providing an opportunity to evaluate the mechanisms connecting these two disorders. The identification of shared disease pathways in BPD and ROP could aid in the development of a single therapy to ameliorate damage to both tissues in preterm infants.








