Abstract
Aims:To evaluate the autonomic modulation and clinical responses during testing of exercise-induced bronchoprovocation (EIB) and when applying non-invasive ventilation (NIV).
Methods:A cross-sectional study that evaluated the heart rate variability at rest, during testing of EIB, in rest after 10 minutes of EIB, and during NIV with bilevel pressure with inspiratory positive airway pressure 12 cmH2O and expiratory positive airway pressure 8 cmH²O. Anthropometric evaluation was performed, examining inflammation and lung function. Clinical control was assessed by questionnaire (Asthma Control Questionnaire 6 [ACQ6]).
Results: A cohort of 55 children aged 10.0±3.3 years were split in response to bronchial provocation test results into a responding group (RG) (n=39) or non-responding group (NRG) (n=16). There was a significant difference between the RG and NRG in clinical control as assessed by ACQ6 (RG=1.16 [0.33–2.0] partially controlled and NRG=0.58 [0.2–1.3] controlled). There was a significant difference in both the time domain and the frequency domain of the heart rate variability, indicating an inhibition of the parasympathetic nervous system during testing of EIB that did not return to baseline levels after 10 minutes and only started to return after the use of NIV in both groups, but the parasympathetic inhibition during the bronchial provocation test in the RG was higher than in the NRG.
Conclusions:The RG presented with worse clinical control in addition to a less effective autonomic regulation. In both groups, NIV assisted in the return of autonomic activity basal levels after the bronchial provocation test.
INTRODUCTION
Asthma is a chronic inflammatory condition characterised by recurrent, reversible episodes of airway obstruction. Medicinal treatment generally involves inhaled corticosteroids and bronchodilators, but clinical control of the condition and an increase in endurance during physical exertion depend on the combination of pharmacological and nonpharmacological treatments.1
Hyper-responsiveness in asthma coexists with inflammation and is associated with an imbalance in the autonomic nervous system2because the cholinergic parasympathetic component is associated with both the pathogenesis of asthma as well as its severity and the inflammatory control.3,4The mechanical force imposed by respiration in the airways is an important modulator of bronchial responsiveness. Deep inspiration reduces responsiveness in healthy adults.5Moreover, continuous positive pressure has been found to cause a substantial reduction in responsiveness in an animal model;6however, short-duration deep inspiration is less effective in individuals with asthma than healthy individuals.7In recent years, noninvasive ventilation (NIV) with positive pressure has been used to improve the outcome of asthma attacks in children8and
adults,9as well as to improve responsiveness in adults when not having an attack. Thus, this method has been touted as potentially beneficial for the treatment of asthma hyper-responsiveness.1NIV is a way of administering intrathoracic positive pressure without the need for a ventilatory prosthesis, and a recent study comparing bilevel and continuous positive airway pressure showed that bilevel was superior for improving bronchial responsiveness and inflammation.10
As individuals with asthma experience both autonomic imbalance and bronchial hyper-responsiveness, positive pressure can minimise these factors.1,8The hypothesis tested in this investigation is that the administration of NIV after exercise-induced bronchoprovocation (EIB) can cause an acute autonomic balance improvement by stimulation of pulmonary stretch receptors and activation of the non-adrenergic, non-cholinergic pathway (NANC), which can be evaluated in parallel with heart rate variability (HRV).2
The aim of the present study was to evaluate autonomic modulation and clinical responses during EIB testing, as well as during the administration of positive pressure after this test.
MATERIALS AND METHODS
Study Design
A cross-sectional study was conducted to determine the effect of noninvasive positive pressure on the airways of children with asthma (when not having an episode) following an EIB test and to evaluate autonomic modulation at different times.
Ethical Aspects
This study was conducted in compliance with the norms for research involving human subjects stipulated in Resolution 466/2012 of the Brazilian National Board of Health and received approval from the human research ethics committee of University Nove de Julho, São Paulo, Brazil, under process number 1487225/2016.
Setting and Sample
This study was developed at the Respiratory Function Evaluation Laboratory located at the Memorial Campus of University Nove de Julho. The participants were recruited from the physical therapy clinic of University Nove de Julho.
Inclusion Criteria
The inclusion criteria were as follows:
Aged 4–16 years.
Not enrolled in any regular physical activity programme.
Diagnosis of asthma based on the criteria of the Global Initiative for Asthma (GINA).
Not having received theophylline or aminophylline and oral corticoids in the previous 30 days.
No occurrence of respiratory infection in the previous 2 months.
Statement of informed consent signed by legal guardian.*
*Statement of informed consent could be signed by children with reading/writing skills aged
≥7 years.
Exclusion Criteria
The exclusion criteria were as follows:
Use of bronchodilator <12 hours prior to evaluation.
Inability to understand or perform any of the tests due to physical or mental impairment.
Intolerance to proposed activities.
Heart disease of an inflammatory, congenital, or ischaemic origin.
Currently experiencing an infectious process with fever.
EVALUATIONS AND TESTS
Anthropometric variables (height, weight, and BMI) were determined. An EIB test was performed. Autonomic modulation and asthma control were evaluated.
Expired Fraction of Nitric Oxide
The fraction of expired nitric oxide (FeNO) was measured following the criteria established by the American Thoracic Society (ATS) in a seated position using the NIOX Mino®equipment (Aerocrine, Uppsala, Sweden). The volunteer was instructed to perform maximum expiration following maximum deep inspiration through the mouth to eliminate any possibility of contamination of this gas.11,12Next, expiration was performed at a constant flow for at least 6 seconds. After 1 minute and 40 seconds, FeNO was recorded from the display of the equipment in parts per billion (ppb). The procedure was performed using a nasal clip to avoid contamination of the air from the nasal cavity.
Lung Function Test
Lung function was tested for the determination of forced vital capacity (FVC) and its derivatives, forced expiratory volume in one second (FEV1) and the FEV1:FVC ratio. The test consisted of maximum inspiratory and expiratory maneuvers, which were performed using a previously calibrated EasyOne®spirometer (ndd Medizintechnik AG, Zurich, Switzerland) until three consistent readings were obtained, based on the recommendations of the ATS. The tests were performed in an air-conditioned environment and Polgar reference values13were adjusted for children for the purposes of classification.
Asthma Control Questionnaire
The Asthma Control Questionnaire 6 (ACQ6) was used to assess the clinical control of asthma and comprises six questions: five addressing symptoms and one addressing the use of a short-lived 1-ß2 agonist as rescue medication. The total score is the mean of the item scores and ranges from 0 (fully controlled) to 6 (uncontrolled) in a period of 7 days. The cutoff point between controlled and uncontrolled asthma is 1.5. The patients were categorised as having controlled asthma (<0.75), partially controlled asthma (0.75–1.50), or uncontrolled asthma (>1.50). The minimally important clinical difference on the six-point scale is 0.50.14,15
Anthropometric Variables
A digital scale (Filizola®, São Paulo, Brazil) was used to determine body weight (kg), and height (m) was measured using a wall stadiometer (Wiso, São José, Brazil) with a precision of 1 mm. These measures were used to determine BMI. The AnthroPlus software program was used to determine the BMI z-scores based on the criteria of the World Health Organization (WHO), which were used to classify the children as either overweight or within the ideal weight range.16
Bronchoprovocation
The EIB test was performed following the guide lines recommended by the ATS17and consisted of a treadmill test with the intensity necessary to reach 80–90% maximum heart rate (HR) (208 – [age x 0.7])18in a climate-controlled room with a temperature of 21°C and relative humidity between 10–15%. The target intensity was reached between 2 and 4 minutes and was maintained for 4–6 minutes. Throughout the test, the volunteer wore a nasal clip and HR was monitored using a heartbeat sensor (Polar V800 [Polar, London, UK]). Peripheral oxygen saturation (SpO2) and any signs of respiratory distress were also monitored. If a child demonstrated intolerance to the test, significant respiratory distress, or a four-percentage point drop in SaO2, the test was interrupted immediately and necessary care was given to ensure the safety and comfort of the child.19
FEV1 was measured five times: 5, 10, 15, 20, and 25 minutes after the test. Bronchoprovocation was considered positive when the reduction in FEV1 was ≥10%.17The severity of bronchoprovocation was assessed based on the percentage of the reduction in FEV1: 10–24% = mild reduction; 25–49% = moderate reduction; and >50% = severe reduction.17
Autonomic Modulation
Activity of the autonomic nervous system was analysed based on HRV, which was determined using a Polar V800 heart meter and analysed in the time and frequency domains. The time domain indices were mean RR interval, standard deviation of all RR intervals, root mean square of differences between adjacent normal RR intervals (root mean square of the successive differences [RMSSD]), percentage of adjacent RR intervals differing >50 ms between one another (pNN50), and the triangular index (total number of RR intervals divided by the maximum height of the histogram of all RR intervals). The frequency domain indices were low frequency (LF: 0.04–0.150 Hz), high frequency (HF: 0.15–0.40 Hz), and the LF:HF ratio.
Readings were performed for 10 minutes in the supine position prior to the bronchoprovocation test (resting HRV), during the test (stable portion with constant velocity), and after the test with the use of positive pressure. The child could not have used a bronchodilator within 2 hours prior to
the readings.20,21
Noninvasive Ventilation
NIV was administered by BIPAP Synchrony (Respironics, Murrysville, Pennsylvania, USA), using a full-face mask (Performax, Bangalore, India) adapted to the size of the child in the sitting position, without the addition of supplemental oxygen (FiO2=21%). There were no problems adapting the children to the interface and all performed the method well.
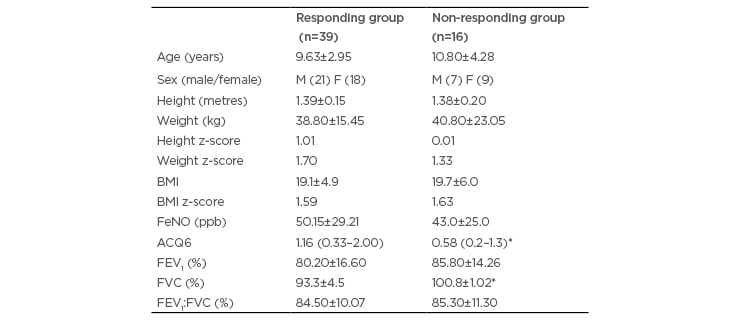
Table 1: General characteristics of the sample.
*p<0.05 in comparison between the responding group and non-responding group.
ACQ6: asthma control questionnaire 6; F: female; FeNO: fraction of exhaled nitric oxide; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; M: male; N: number.
Pressure levels were chosen based on a previous study of the group: inspiratory positive airway pressure was 12 cmH2O and expiratory positive airway pressure was 8 cmH2O.10The NIV was applied for 10 minutes 25 minutes after EIB.
STATISTICAL ANALYSIS
The Shapiro–Wilk test was used to determine the distribution (normal or non-normal) of the data. The non-paired Student’s t-test was used to compare the groups. Analysis of variance (ANOVA) and Tukey’s post hoc test were used for the intra-group analyses of HRV and FEV1. The Mann–Whitney test was used for the comparison of non-parametric data, such as the ACQ6.
The Minitab 14 program was used for the statistical analysis and the data were expressed as the mean and standard deviation. The level of statistical significance was set at 5% (p≤0.05).
The sample size was calculated using a standard deviation of 19 for HF during NIV described in a study by De Freitas Dantas Gomes et al.,8with a 10-point minimum difference to be detected, an alpha of 0.05, and beta of 0.90. The minimum number of participants was established to be 38 asthmatic children.
RESULTS
The study evaluated 55 children with a diagnosis of asthma based on the criteria of GINA and eosinophilic inflammation (FeNO). Table 1 displays the general characteristics of the sample, which was divided based on the results of the bronchoprovocation test into a responding group (RG) (n=39; 70% of the sample) and non-responding group (NRG)
(n=16; 30%). The RG was subdivided based on the severity of the response: mild, moderate, and severe. Moreover, HRV was analysed four times: 1) at rest before the bronchoprovocation test; 2) at the peak of the test at a stable velocity on the treadmill; 3) at rest 10 minutes after the test; and 4) during NIV with positive pressure.
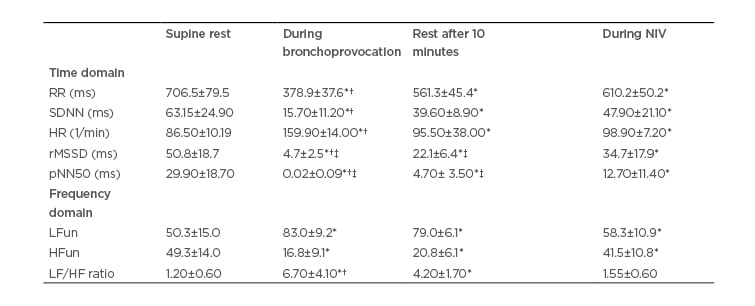
Table 2: Heart rate variability data in the responding group.
Data expressed as mean±standard deviation
*p<0.05 in comparison to pretest resting condition; †p<0.05 in comparison to noninvasive ventilation; ‡p<0.05 in comparison to non-responding group.
HF: high frequency; HR: heart rate; LF: low frequency; NIV: noninvasive ventilation; pNN50: percentage of difference between adjacent normal R–R intervals >50 ms computed over entire height of histogram created by charting all R-R intervals; rMSSD: square mean root of sum of squared differences between iRR; RR: R–R interval; SD: standard deviation; SDNN: mean standard-deviation of all iRR.
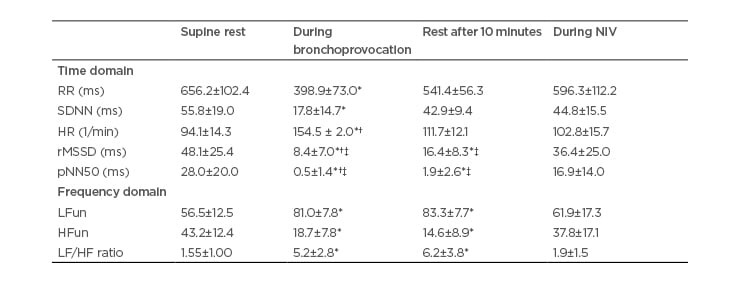
Table 3: Heart rate variability data in the non-responding group.
Data expressed as mean and standard deviation.
*p<0.05 in comparison to pretest resting condition; †p<0.05 in comparison to noninvasive ventilation; ‡p<0.05 in comparison to non-responding group.
HF: high frequency; HR: heart rate; LF: low frequency; NIV: noninvasive ventilation; pNN50: percentage of difference between adjacent normal R–R intervals >50 ms computed over entire height of histogram created by charting all R-R intervals; rMSSD: square mean root of sum of squared differences between iRR; RR: R–R interval; SD: standard deviation; SDNN: mean standard-deviation of all iRR.
The results of the ACQ6 revealed that the RG had partially controlled asthma and the NRG had controlled asthma. FVC percentage was significantly lower in the RG than the NRG, although the values were within the range of normal in both groups. The BMI z-score revealed that both groups were in the ideal range (between +2 and -2). Both groups exhibited very high FeNO (>35 ppb): very high FeNO was found in 34 of the 39 patients (87.17%) in the RG and 11 of the 16 patients (68.75%) in the NRG.
FEV1was measured prior to the bronchoprovocation test, as well as five times after the test. A significant reduction was found 5 and 10 minutes after the test, with recovery close to the baseline level beginning at 15 minutes after exercise-induced bronchoconstriction.
Regarding the severity of airway obstruction among the 39 patients in the responding group during the bronchoprovocation test, 18 (46.15%) exhibited a mild reduction in FEV1 (10–24%), 16 (41.02%) exhibited a moderate reduction (25–49%), and 5 (12.8%) exhibited a severe reduction.
Tables 2 and 3 respectively display the HRV indices in the time and frequency domains for the RG and NRG.
In both groups, an increase in sympathetic activity occurred during the EIB test (HR and LF) and a partial return of parasympathetic activity occurred at rest 10 minutes after the test, but without returning to baseline levels (RMSSD, pNN50, and HF). Thus, the sympathetic–vagal balance had been altered, as demonstrated by the LF:HF ratio. The return to baseline levels only occurred during the administration of NIV, as shown by the significant differences in all variables in the time and frequency domains shown in Table 2. The RG demonstrated more accentuated reductions in pNN50 than the NRG during the EIB test. Moreover, the return of these same variables at rest 10 minutes after the test also occurred in a more accentuated manner in the responding group.
DISCUSSION
Exercise-induced asthma is found in 40–90% of children with asthma22and is considered the main reason for a reduction in physical performance in this population23because the emergence of this condition leads to a reduction in the intensity of physical activity and an increase in sedentary behaviour. This aspect contributes to poorer clinical control of asthma, which further aggravates exercise-induced asthma, leading to a cycle that is difficult to break.24This was confirmed by the present findings, as 70% of the sample had a positive bronchoprovocation test and the RG also had poorer clinical control (partially controlled asthma in contrast to the controlled asthma in the NRG).
Concerned with the efficacy of the bronchoprovocation test, the authors performed the test for a prolonged period of time (up to 25 minutes). Significant reductions in FEV1 were found at the 5thand 10thminutes. This methodological care regarding the time of the test enabled the asthmatic children to have a positive response to the bronchoprovacation test independently of whether or not they were responsive. Johansson et al.25found a greater reduction in FEV1 in the 10thminute in most patients. Clinical control of asthma and the absence of children with obesity were also important, as such aspects could have affected the results, as described by Ostrom et al.26Moreover, the lack of differences between sexes agrees with similar findings reported by Johansson et al.25
The inflammatory process was assessed based on FeNO. Grzelewski et al.27established FeNO >16 ppb as an independent risk factor for the development of exercise-induced asthma. In the present sample, FeNO was high (around 50 ppb) in both groups, but the percentage of patients with FeNO >35 ppb was higher in the RG than the NRG. Moreover, the combination of other factors, such as the action of the autonomic nervous system, which is altered in patients with asthma, may also have exerted an influence.
Parasympathetic activity is predominant in individuals with asthma. This activity is directly proportional to the severity of the condition3and leads to an abnormal response of the autonomic nervous system. Despite inadequate clinical control, the patients in the present study were classified as having mild-to-moderate severity and, based on the autonomic nervous system at rest, demonstrated a balance between the sympathetic and vagal components, represented by the HF and LF indices. The parasympathetic component performs a dual function in the activity of the bronchial smooth muscles. As sympathetic fibres only innervate the bronchial vasculature, the bronchoconstriction and bronchodilation mechanisms depend on which parasympathetic component is predominant and what stimulus triggered the mechanism.2
Preganglionic parasympathetic fibres stem from the vagus nerve and are projected to the airways with cholinergic synapses, whereas postganglionic fibres are from the NANC component and are directed to the innervation of the bronchial smooth muscles, glands, and microvasculature. The bronchial muscle constriction and dilation response depends on the balance between the excitatory pathway (NANCe) with neurotransmission performed by neuropeptides and bradykinin, which cause bronchoconstriction, oedema, and the production of mucus, and the inhibitory pathway (NANCi), which is the only neural pathway that causes bronchodilation, and the neurotransmitters involved in this process are nitric oxide (NO) and vasoactive
intestinal peptide.2
The NANC pathway is dysfunctional in asthmatic patients, which becomes evident when there is a need for the action of this bronchodilating pathway due to exposure to physical exertion. The children in the RG seemed to have less stable modulation than those in the NRG, as demonstrated by the considerable fluctuation in the parasympathetic component during effort, which varied in intensity more than in the NRG and likewise returned to normal in a more accentuated manner. One of the hypotheses for this ‘maladjustment’ is the predominance of the NANCe pathway and reduction in the activity of the NANCi pathway.2,4,27The main reason for the inhibition of the NANCi pathway in asthmatic individuals is the reduction in NO activity caused by persistent inflammation of the airways, which reduces the activity of the neurotransmitter neuronal NO and stimulates the production of the induced isoform (induced nitric oxide synthase), which increases oedema and secretion, perpetuating inflammation, and is measured in the exhaled air in the form of FeNO, which was very high in both groups of the present study, but in a greater number of the children in the RG.
Changes in the autonomic nervous system which affect airway control can be visualised through an analysis of HRV, as vagal cardiac reactivity is greater in asthmatic individuals than healthy individuals. Reactivity seems to have been manifested more intensely in the RG, which demonstrated a faster decline of the parasympathetic system during the EIB test as well as a faster return in the 10thminute after the test. The magnitude of vagal cardiac reactivity is related to the severity and control of asthma,28which explains the greater instability of this component in the responding group during the EIB test, as demonstrated by HRV. The clinical importance of gaining a better understanding of HRV behaviour in asthmatic children underscores the need for further studies on autonomic modulation and bronchial hyper-responsiveness in this population.
The HRV indices tended to return to baseline levels only during the administration of NIV. The mechanical stretching of the airways is an important modulator of bronchial reactivity, as demonstrated in adults1and animals, as well as during asthma attacks in children.8The stretching of the airways with NIV stimulates pulmonary stretch receptors that activate the NANCi pathway and consequently promote bronchodilation, leading to a better adjustment of the autonomic nervous system. This is likely a factor that contributes to the reduction in FeNO.
The use of non-pharmacological measures in asthma is important to the treatment of a chronic illness that needs to be controlled constantly. According to Busk et al.,1NIV is a non-pharmacological therapy for the treatment of bronchial reactivity. Thus, further clinical trials are needed to evaluate the possible effects of this method in asthmatic children when not in the throes of an attack.
The limitations of the study are the same limitations of the HRV, which reflect the behaviour of the autonomic nervous system seen by cardiovascular reactions and indirectly infer the dynamics of the airways especially the smooth muscles of the bronchi.
CONCLUSION
Besides the accentuated reduction in FEV1 in the RG in the 5thand 10thminutes, a change in autonomic modulation occurred in both groups when they underwent the EIB test, with greater vagal cardiac instability in the RG than the NRG and the return of HRV indices close to baseline values only with the administration of NIV with bilevel pressure. The findings confirm the influence of this therapeutic resource on autonomic modulation in children with asthma.








