Abstract
Bronchial asthma is the most common chronic disease in pregnancy associated with adverse pregnancy, obstetric, and perinatal outcomes. The aim of this study was to determine the influence of the steps of asthma treatment during pregnancy on adverse pregnancy, obstetric, and perinatal outcomes. The data of all women with singleton delivery in 2011–2017, including the diagnosis of asthma and its treatment for the same woman, were obtained from the National Registry of Reimbursed Health Services (NRRHS) of the Czech Republic. Relation of asthma and the steps of treatment to pregnancy, labour, and perinatal outcomes taken from the National Register of Reproduction Health (NRRH) for the period 2011–2015 were analysed using logistic regression and described by odds ratios, 95% confidence interval, and statistical significance. Of the total number of 752,000 women with singleton delivery, asthma and/or its treatment were found in 6.27% of deliveries. Data from 460,324 births, in which the combination of data sources was available, showed the association between asthma and pre-eclampsia, caesarean section, and birth weight ≤2,500 g, only for the fifth step of treatment (p<0.001). Caesarean section was more frequent in all evaluated groups of treatment compared with women without asthma (p<0.001). Gestational age of <37 weeks was found in children of mothers with asthma diagnosis and no treatment and for women at the fifth step of treatment (p=0.003). The incidence of birth defects and Apgar scores of <7 in 5 minutes were without statistical significance in all evaluated women. The authors concluded that pregnant women with asthma are at risk from adverse pregnancy, obstetric, and perinatal outcomes, especially upon the fifth stage of treatment.
INTRODUCTION
Asthma is one of the most common chronic diseases in pregnancy, with a reported prevalence of 3.7–12.0%.1-7Maternal asthma severity and control are often associated with pregnancy complications, adverse labour, and perinatal outcomes. A number of papers, meta-analyses, and population-based studies have been published on this topic.2,6Hypertension, gestational diabetes mellitus, pre-eclampsia, vaginal bleeding, complicated delivery (increased birth rate by caesarean section), fetal growth restriction, low birth weight, premature birth (<37thweek), neonatal hypoxia, and increased perinatal mortality have been observed more frequently in pregnant women with uncontrolled asthma.1,3,6,8,9Women with well controlled asthma have a much lower risk of these adverse complications.8
According to the Global Initiative for Asthma (GINA) guidelines, management of asthma in pregnancy is based on disease control and prevention of exacerbations that are achieved by pharmacological and non-pharmacological treatment. Pharmacological treatment uses three basic groups of drugs: relievers, controllers, and add-on therapy. In recent years, biological therapy has been recommended for patients with severe asthma.10The GINA guidelines use a stepwise approach to asthma treatment and recommend the use of short-acting β2-agonists (SABA) as a relief treatment for all steps of asthma. Regular administration of low doses of inhaled corticosteroids (ICS) is recommended from the second step of treatment. In steps 3 and 4, moderate or high doses of ICS and long-acting β2-agonists (LABA) are recommended. Steps 4 and 5 require add-on treatment with leukotriene receptor antagonists (LTRA), theophylline, and/or tiotropium bromide. The fifth step recommends the use of biological therapy. Adding low doses of oral corticosteroids is another option.11The disease severity is therefore determined by the steps of the treatment that are needed to control asthma and to prevent exacerbations.2,5,6,10
Additionally, pharmacological treatment of asthma in pregnancy is guided by categories of treatment safety, of which the U.S. Food and Drug Administration (FDA) pregnancy categories are the best known. The classification system uses five categories (A, B, C, D, and X) and is based on the degree of risk of teratogenic effects on the fetus.11,12In the case of asthma treatment, no drug group falls into the FDA category A; therefore, drugs are chosen from the FDA category B when the benefit outweighs the risk. The FDA category B includes budesonide, terbutaline, montelukast, ipratropium bromide, and omalizumab. The remaining asthma medications are included in the FDA category C: salbutamol, salmeterol, formoterol, vilanterol, and other ICS apart from budesonide (beclometasone dipropionate, fluticasone propionate, fluticasone furoate, mometasone furoate), theophylline, and systemic corticosteroids.12
The aim of this study was to show how the severity of the treatment, which is given by the number of medications needed to control asthma, affects complications during pregnancy and labour as well as perinatal outcomes. Unlike previous studies, which have worked with the severity of asthma defined by clinical symptoms, lung function, and the need to use relief medication, this study includes the severity of asthma determined by the steps of treatment used. Another aim of the study was to find out which drug groups were most commonly prescribed during pregnancy, and whether their selection respected the safety pregnancy categories.
METHODS
The analysis used data from national health registries of the Czech Republic. These registries contain data on the level of individual patients, with each patient being identified by a pseudonymous code that makes it possible to combine all records on a particular patient but not to identify the patient. The National Registry of Reimbursed Health Services (NRRHS) contains data on all healthcare paid by the public health insurance (almost 100% of healthcare in the Czech Republic) and the data is available for the period 2010–2017. It was adopted for the primary identification of deliveries using procedure codes for vaginal delivery, caesarean section, and other types of delivery, as well as for the identification of asthma and its treatment during pregnancy from its onset; the ICD10 code J45 was adopted for the identification of asthma and the Anatomical Therapeutic Chemical (ATC) classification code R03 (without CHOPN treatment codes) and codes H02AB04, H02AB07, and H02AB09 (in combination with the diagnosis) for the identification of its treatment. A combination of drugs according to asthma treatment guidelines was adopted for the identification of asthma treatment steps. The information on asthma in pregnant women from NRRHS was combined with detailed information on singleton deliveries from the National Register of Reproduction Health (NRRH); the connections on patient level between NRRHS and NRRH are available for roughly 90–95% of deliveries each year (NRRHS and NRRH use different pseudonymous code and the links between them are missing in approximately 5–10% of patients). NRRH data are available until the year 2015 and the analysis was computed for the period 2011–2015; the year 2010 is not included due to the non-availability of treatment data in 2009, when some pregnancies started.
Standard descriptive statistics were applied in the analysis; absolute and relative frequencies were used for categorical variables. The relation of asthma to perinatal outcomes was analysed using logistic regression and described by odds ratio (OR), 95% confidence interval (CI), and statistical significance. The analysis was carried out using the SPSS 25.0.0.1 (IBM Corporation, 2018).
Using data from the national health registers of the Czech Republic, the authors evaluated 752,000 deliveries of women with singleton births in the period 2011–2017 and divided these deliveries into groups based on the presence of asthma and its treatment (Table 1).
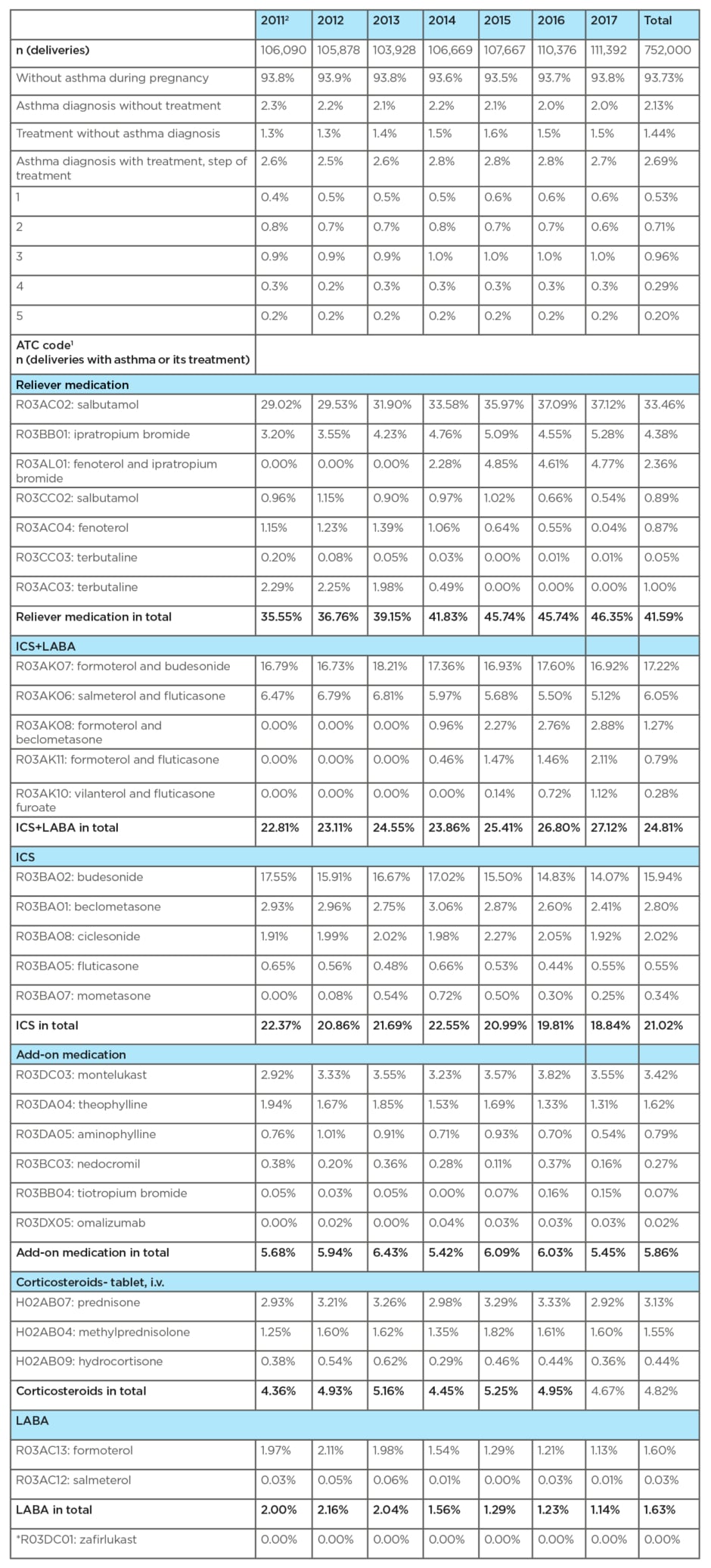
Table 1: Asthma diagnosis and/or its treatment reported during pregnancy.
1 Patients with asthma diagnosis or its treatment reported during pregnancy in National Registry of Reimbursed Health Services (NRRHS).
2 Year 2010 not shown (some pregnancies started in 2009 and data of 2009 are not available).
*Drug is not available on the Czech market.
ICS: inhalded corticosteroids; iv: intravenous; LABA: long-acting β2-agonists.
Variables on maternal age, educational level, marital status and parity, smoking, and gestational diabetes mellitus were retrieved from the NRRH (Table 2). Based on reported ACT codes, drug groups for asthma that had been prescribed to women during their pregnancy, and delivery in 2011–2017 were identified: relievers, controllers, and add-on therapy (Table 1).
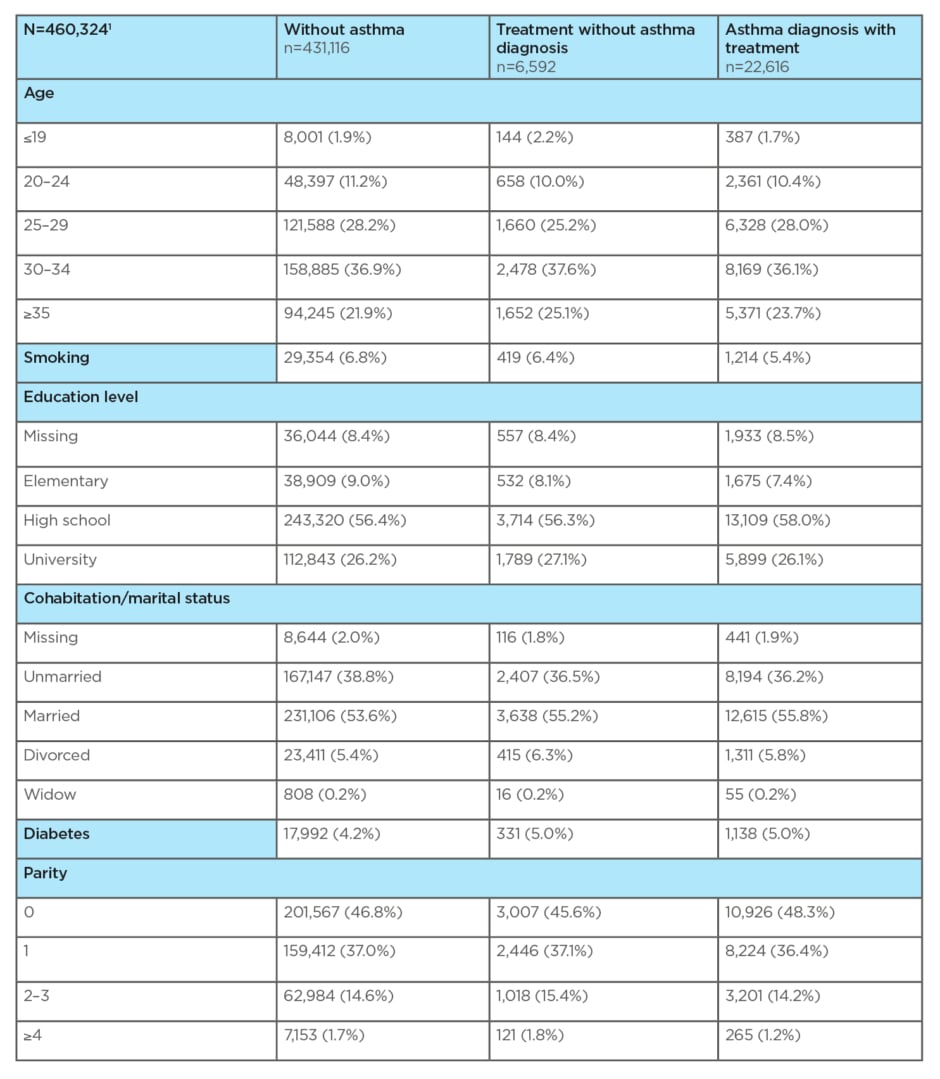
Table 2: Characteristics of analysed population according to bronchial asthma.
1 Analysis computed for the singleton deliveries in period 2011–2015; year 2010 is not included due to non-availability of treatment data in 2009 when some pregnancies started; combined data of National Registry of Reimbursed Health Services (NRRHS) and National Register of Reproduction Health (NRRH) are available until 2015 only, connection between NRRHS and NRRH available for 90–95% of deliveries each year.
Statistical data on the perinatal outcomes of singleton deliveries were only available in the combined data of NRRH and NRRHS from the period 2011–2015. This group of 460,324 deliveries was divided into 4 subgroups: 1) deliveries by women without asthma (n= 431,116); 2) deliveries by women without asthma diagnosis and with asthma treatment (n=6,592); 3) deliveries by women with asthma diagnosis and with asthma treatment (n=12,502); and 4) women with an asthma diagnosis and without treatment (n=10,114) (Table 2). Statistical data on complications in pregnancy (pre-eclampsia or eclampsia), in the course of delivery (caesarean section) and the adverse perinatal outcomes (Apgar score in 5 minutes <7, birth weight <2,500 g, gestational age <37 weeks, and congenital malformations) were found for groups 1–3 of deliveries. The fourth group of deliveries, i.e., those by women with asthma diagnosis and without treatment, is not included in Table 3.
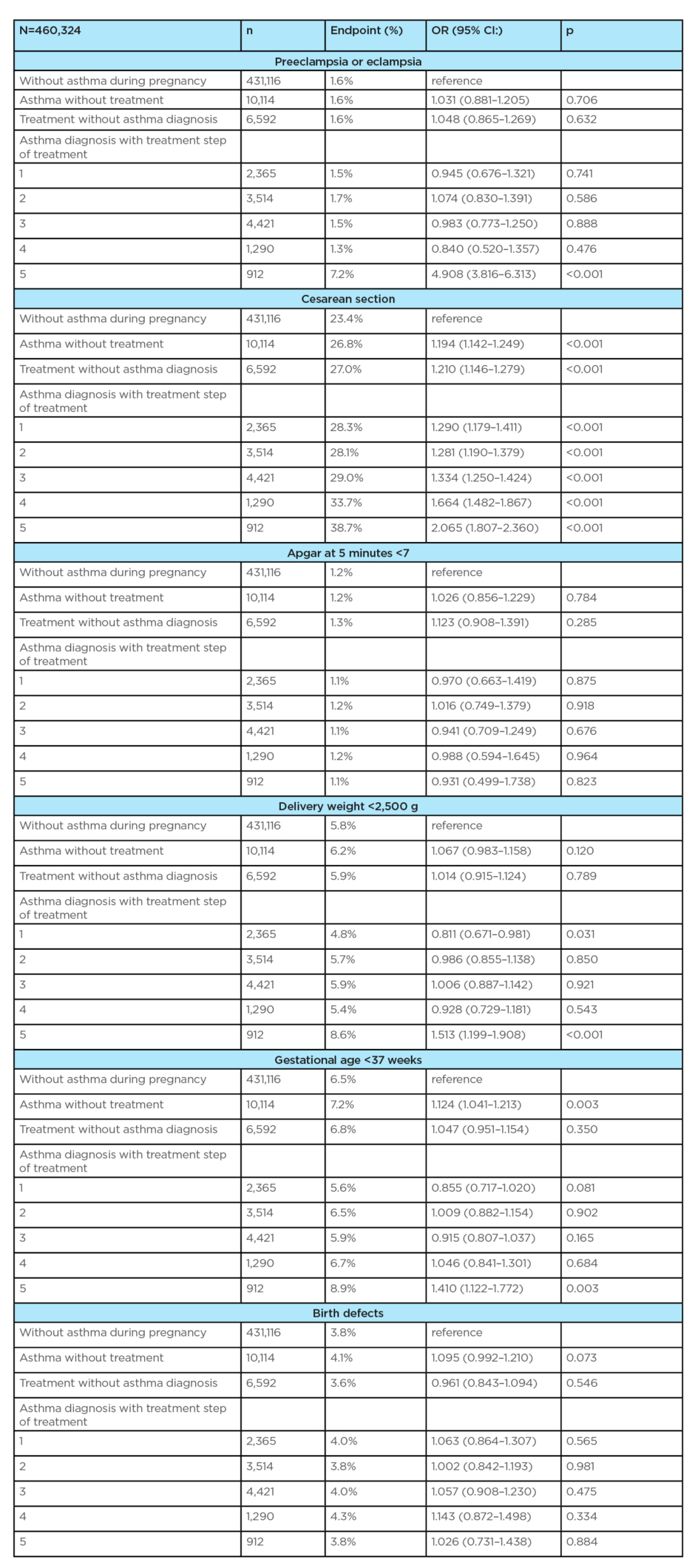
Table 3 continued.
1 Analysis computed for the period 2011–2015; year 2010 is not included due to non-availability of treatment data in 2009 when some pregnancies started; combined data of National Registry of Reimbursed Health Services (NRRHS) and National Register of Reproduction Health (NRRH) are available until 2015 only, connection between NRRHS and NRRH available for 90–95% of deliveries each year.
CI: confidence interval; OR: odds ratio.
RESULTS
Two data sets were created from records on registered singleton deliveries. In the first data set, from the period 2011–2017, a total of 752,000 deliveries were evaluated. Based on the diagnosis and treatment, these deliveries were divided into three subgroups: 1) deliveries by women with diagnosed asthma and with asthma treatment (2.69% on average); 2) deliveries by women with diagnosed asthma and without treatment (2.13% on average); and 3) deliveries by women without the diagnosis of asthma and with asthma treatment (1.44% on average). On average, asthma diagnosis and/or its treatment were found in 6.27% of deliveries. Of the total number of deliveries evaluated, the group of women without the diagnosis of asthma and without asthma treatment accounted for 93.73% on average (Table 1).
The severity of asthma in pregnancy was determined by the number of drug groups (i.e., the step of treatment) used to control asthma and to prevent exacerbation. In this group of deliveries, most women with asthma were treated with the third step of treatment (0.96%), followed by women treated with the second step (0.71%), the fourth step (0.29%), and the fifth step (0.20%) of treatment.
The frequency prescription of individual drug groups for the treatment of asthma in deliveries in the period 2011–2017 are shown in Table 1. Reliever medications were the most commonly prescribed drugs (41.59%), with salbutamol as the most frequent (33.46%). ICS/LABA combination products were the second most commonly prescribed drugs (24.81%), mostly budesonide/formoterol (17.22%). ICS monotherapy was third (21.20%), with budesonide as the most commonly prescribed drug from this group (15.94%). Montelukast (3.42%) was the most commonly prescribed drug from the add-on therapy group (3.42%).
When monitoring trends in prescribing asthma medications, an increase in salbutamol prescription was observed from 29.02% in 2011 to 37.12% in 2017. As for combination products, a slight increase in prescriptions was observed (from 22.81% in 2011 to 27.12% in 2017) at the expense of ICS monotherapy (from 22.37% in 2011 to 18.84% in 2017). The LTRA prescription increased from 2.92% in 2011 to 3.55% in 2017. An increase was also observed for tiotropium from 0.05% in 2011 to 0.15% in 2017. Prescription of systemic corticosteroids remained at approximately the same level for the entire 7-year period (from 4.36% in 2011 to 4.67% in 2017). Omalizumab was rarely prescribed (0.02% on average) and mepolizumab was not available on the Czech market until 2017.
The second data set available to evaluate the perinatal outcomes, from the years 2011–2015, involved 460,324 deliveries. The diagnosis of asthma without treatment was found in 10,114 (2.2%) deliveries. Asthma treatment without a previous diagnosis was found in 6,592 (1.4%) deliveries. Asthma diagnosis and its treatment according to the disease severity was found in 12,502 (2.7%) deliveries. Altogether, these groups involved 29,208 (6.4%) deliveries. Neither asthma nor its treatment were reported in 431,116 (93.7%) deliveries. Age (30–34 years) was almost the same for all of the aforementioned groups (Table 2). The proportion of smokers was lower in women with asthma diagnosis and treatment (5.4%) as compared to women with asthma treatment but without a previous diagnosis (6.4%) and also to women who did not suffer from asthma (6.8%). Education of only elementary level was more frequently seen in patients without asthma (9.0%). Marital status and parity were comparable for all groups. The incidence of gestational diabetes mellitus was higher in women with asthma treatment (5.0%), both with and without diagnosis, compared to women without asthma treatment (4.2%) (Table 2). Association between asthma in pregnancy and complications in pregnancy (pre-eclampsia/eclampsia) and adverse perinatal outcomes (birth weight <2,500 g) was statistically significant only for the fifth step of asthma treatment (OR: 4.908; 95% CI: 3.816–6.313; p<0.001, and OR: 1.513; 95% CI: 1.199–1.908; p<0.001, respectively). Gestational age <37 weeks was statistically more significant in women with asthma who were not treated (OR: 1.124; 95% CI: 1.041–1.213; p=0.003) and for women in the fifth step of asthma treatment (OR: 1.410; 95% CI: 1.122–1.772; p=0.003).
Delivery complications (caesarean section) were statistically significantly more frequent in all groups of interest (i.e., in women with asthma diagnosis and treatment regardless of treatment steps, in women with asthma diagnosis who were not treated and in women without asthma diagnosis but with asthma treatment) when compared to women without asthma (p<0.001 in all cases; OR from 1.194 in asthma without treatment to 2.065 in the fifth step of treatment). The incidence of congenital malformations and Apgar score at 5 minutes <7 had no statistical significance for all of the above-mentioned groups of interest and did not differ between women with and without asthma and its treatment (Table 3).
DISCUSSION
This study evaluated two groups of deliveries. In the analysis of the first group, asthma diagnosis without asthma treatment was found in 2.13% deliveries, whereas asthma treatment without asthma diagnosis was reported in 1.44% of deliveries.
In a Finnish population-based study from 2018, Kemppainen et al.13reported that the diagnosis of asthma without asthma medication was found in 0.8 % of women. In a Swedish population-based study, Rejnöet al.1 reported that 0.4 % of women were taking asthma medication without a diagnosis of asthma.
This present study also evaluated the steps of asthma treatment based on the findings of drug groups that were prescribed during pregnancy. The third step of treatment was most frequently used in the study’s group of interest.
In a study from 2013, Charlton et al.14used longitudinal electronic medical records, which were associated with prescribing data, and assessed the degree of asthma treatment during pregnancy based on the prescription of asthma medications. Data were obtained from the General Practice Research Database (GPRD) in the UK. Most women were treated with the first step of treatment.14
In a Dutch population-based study, Zetstra-van der Woude et al.15 reported that 8.1% of women took asthma medication with at least one drug. Of this group, 33.9% had prescription for SABA only.15 According to Lim et al.,16almost one-third of pregnant women discontinue or reduce their asthma preventing drugs during pregnancy and overcompensate with SABA. In 2016, Carlton et al.17published a study to determine the prescription of asthma medications during pregnancy, which used data from seven electronic databases from Denmark, Norway, the Netherlands, Italy (Tuscany and Emilia-Romagna), Wales, and from a database of general practitioners from the rest of the UK.17 SABA was prescribed to 90% of women in the UK, 75% of women in Denmark and Norway, and 26% of women in Italy. ICS during pregnancy was prescribed to 50% of women in Norway, 60% of women in the UK and Denmark, and 89% of women in Emilia-Romagna. In the UK (including Wales) and Italian databases, beclometasone was the most commonly prescribed ICS, whereas budesonide was most commonly prescribed in Denmark, and beclometasone and budesonide were equally prescribed in Norway. Norway was the only region where the prevalence of ICS prescription in a fixed-dose combination with a LABA was higher than the prescription of ICS products in monotherapy. Norway and Italy were the only countries, however, where the guidelines recommended budesonide as the ICS of choice, and Denmark was the only region where budesonide was found to be the most commonly prescribed ICS. In Italy, despite the guidelines recommending budesonide, beclometasone was by far the most commonly prescribed ICS. During pregnancy, evidence of a reduction in the prescription of LABA, both alone and as part of a fixed-dose combination with ICS, was observed in Norway, the Netherlands, and Italy. The percentage of LTRA prescription was low, from 0.04% in Norway to 0.41% in Wales. No prescription of anticholinergic drugs was reported in Denmark, whereas in the Netherlands, these were prescribed to 0.16% of women. Prescription of oral corticosteroids was highest in the UK and Italy. Prednisolone was the most commonly prescribed oral corticosteroid in all regions. During the 7-year study period, in Denmark, Norway, and the UK, the prevalence of prescripted LABA in fixed combination with ICS increased while pregnancy prescriped LABA decreased.17
This study respects the categories of drug safety in pregnancy, because ICS budesonide (FDA-B), which was used in ICS monotherapy and also in fixed combination with ICS (ICS/LABA), was still the most commonly prescribed drug. From the add-on therapy, montelukast (FDA-B) was the most commonly chosen drug, and an increasing trend in its use has been observed (Table 1). Several prescription trends were observed in the 7-year period. A decrease in the prescription of ICS monotherapy was reported, in favour of the use of fixed combinations (ICS and LABA), with a predominance of budenoside; moreover, an increase in the prescription of LTRA (FDA-B) was observed, as well as an increase in SABA prescription. The prescription of systemic corticosteroids (most commonly methylprednisolone) has not changed.
In the 7-year study period of prescribing habits in Europe, a similar increasing trend in the prescription of fixed combinations of ICS and LABA was found in Denmark, Norway, and the UK.17
In the second group, the incidence of pregnancy complications, childbirth, and adverse perinatal outcomes in relation to asthma treatment was assessed. In this group of deliveries by women with asthma, there was a statistically significant (p<0.001) incidence of some adverse outcomes (pre-eclampsia/eclampsia, caesarean section, birth weight<2,500 g, gestational age of <37 weeks) only in women treated with the fifth step of asthma treatment, i.e., those with severe asthma. The above-mentioned results are consistent with a number of studies, meta-analyses, population-based studies, and consensus studies.1,2,6,8,9,12In a Finnish study from 2018, it was shown that the number of used asthma medications increased the risk of low birth weight and fetal growth restriction. Each medication group increased the risk of low birth weight by 6% (OR: 1.06; 95% CI: 1.00–1.13; p=0.0365) and fetal growth restriction (SGA) by 13% (OR: 1.13; 95% CI: 1.07–1.19; p<0.0001).13The risk of perinatal mortality or preterm birth, however, was not increased in the logistic manner of evaluation.13A Swedish study showed that maternal asthma was associated with an increased risk of almost all pregnancy complications. There were also increased odds for adverse birth outcomes, including a low birth weight and a low gestational age.1In this study of all groups of deliveries (women with and without asthma diagnosis, with and without asthma treatment), there was no evidence of a higher incidence of congenital malformations, even for women with severe asthma, as compared to women without asthma. Systematic reviews and meta-analyses of >56,000 pregnancies with asthma and >1 milion pregnancies without asthma, from 14 studies, found a significant but very small increase of congenital malformations among women with asthma as compared with women without asthma (risk ratio: 1.30; 95% CI: 1.02–1.21).9However, the European case-malformed control study (EUROmediCAT) of 13 registers showed that cleft palate (OR: 1.63; 95% CI: 1.05–2.52) and gastroschisis (OR: 1.89; 95% CI: 1.12–3.20) were significantly more frequent in women who were treated in the first trimester by β2-agonists.7An exploratory analysis found an association between renal dysplasia and exposure to the combination of ICS/LABA (OR: 3.95; 95% CI: 1.99–7.85). Administration of ICS during the first trimester of pregnancy appeared to be safe in relation to the risk of a range of specific major congenital anomalies.7In this present study group, deliveries led by caesarean section were significantly more frequent (p<0.001) in all groups of deliveries, as compared to women without asthma (Table 3).
A number of multicentre studies and meta-analyses have shown that women with asthma have a higher risk of caesarean delivery and even twice the risk of elective caesarean section (risk ratio: 2.14; 95% CI: 1.16–3.95) than women without asthma.9
A Swedish population-based study linked a higher incidence of caesarean delivery in women with asthma with the possibility that women with chronic conditions generally undergo this surgical procedure for medical reasons because doctors assume that vaginal delivery is associated with more complications.1Prof Michael Schatz, one of the leaders of international recommendations for the management of asthma during pregnancy, indicates that adverse perinatal outcomes in women with asthma might be explained by general pathogenetic factorsthat predispose the patients to bronchial, vascular, and uterine muscle hyperresponsiveness, as well as circulating mediators that cause abnormalities of smooth muscles and of the autonomic nervous system.12
CONCLUSION
Asthma during pregnancy is associated with increased risks of pregnancy, delivery, and perinatal complications. Severe asthma, defined by the number of drugs that a pregnant patient with asthma needs to control the disease and to prevent exacerbations, was more often associated with adverse outcomes. The prescription profile of drugs used in asthma during pregnancy should be consistent with international recommendations in which the choice of drugs is guided by safety categories. Appropriate asthma care during the preconception period, correct determination of treatment steps during pregnancy, as well as a proactive approach to treatment will help reduce potential adverse effects on pregnancy, delivery, and perinatal outcomes.








