Abstract
Background: As the severe acute respiratory syndrome coronavirus 2 era commenced, a new entity was added to the already hefty bulk of parenchymal lung diseases in post-COVID-19 pulmonary fibrosis. A wide range of findings from mild ground glass opacities to exuberant fibrosis are seen on high resolution CT of the thorax. However, the authors came across a pattern that was frequently repeated, and therefore conducted an observational study on the radiological findings.
Method: The study was conducted for a period of 6 months in the departments of Respiratory Medicine and Radiodiagnosis at King George’s Medical University, Lucknow, India. The radiological findings on high resolution CT thorax of consecutive patients who reported to the Department of Respiratory Medicine after recovering from COVID-19, and were previously reverse transcriptase-PCR-positive or serologically confirmed, were studied.
Result: There were a total of 56 subjects (32 males; mean age: 56 years). The most common finding was ground glass opacities (89%). Reticulations were seen in 86% of patients, with a unique dome-shaped fibrosis parallel to pleural surface in 54%, patchy consolidation in 49%, and scattered cysts in 43%. The distribution was mostly bilateral with slight predominance of lower lobes (57%).
Conclusion: Ground glass opacities, reticulations, and consolidation are fairly common in patients with pulmonary sequelae of COVID-19. It has a peculiar predilection for involvement of subpleural space with cupola or band-shaped fibrosis.
Key Points
1. Parenchyma of the lungs can undergo fibrosis due to a number of known aetiology and is associated with a poor outcome.2. This observational study of 56 subjects highlights the most common complaints in post-COVID-19 pulmonary fibrosis, which includes fatigue, coughing, and dyspnoea.
3. The authors used high resolution CT to study the different radiological findings in post-COVID-19 pulmonary fibrosis and indicate that it should be considered in the aetiology of fibrotic lung diseases.
INTRODUCTION
The parenchyma of the lungs can undergo fibrosis due to known aetiology such as radiation or drug induced fibrosis; occupational or connective tissue related interstitial lung disease; or it may have genetic basis or remain idiopathic, as in idiopathic pulmonary fibrosis. Over 200 interstitial lung diseases are known, and repeated attempts are made to reclassifying them as understanding improves.1 The latest classification proposed by Cottin et al.1 was based on their progression to fibrosis. Those with tendency to have a fibrotic course are associated with a poor outcome. A new undesirable addition to this is post-COVID-19 pulmonary fibrosis and the rapidity of development of lung scarring.2
When the world was jostled by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in December 2019, little was known that the aftermath would be so long-lasting. Both virus-mediated cytopathic effects and host immune response causing cytokine storm are blamed for the pulmonary sequelae. The inflammatory milieu of the lungs with high levels of TNF-α; IL 6, 8, and 12; and interferon-γ cause tissue damage, with subsequent healing and evolvement into widespread fibrosis.2,3 Other likely mechanisms include angiotensin-induced pulmonary vasoconstriction and activation of pro-fibrotic molecules such as tumour growth factor β1, ventilator-induced lung injury, and oxygen toxicity.2,4 Changes were more pronounced in males, the elderly, those with comorbidities, chronic alcoholics, smokers, those with severe disease, and those requiring mechanical ventilation.5-9 The disease not only wreaked a new havoc of its own, but also affected the course and epidemiology of other disorders by producing an intense inflammatory state, as well as afflicting healthcare services.10-12
Certain characteristic radiological findings were identified in patients with COVID-19, and CT scans of the thorax played a crucial role not only in diagnosis but also in assessing severity. The second week of illness was more commonly associated with the development of radiological abnormalities.13-16 Multiple scoring systems such as Coronavirus Disease 2019 Reporting and Data System (CO-RADS) and CT severity score (CSS) were developed.17,18 In this study, out of the typical findings described in CO-RADS 5, such as crazy paving, consolidations, and the subpleural bands, fibrotic bands were seen to persist long in nearly half of subjects long after they became normoxic. The most frequent remnants were ground glass opacities and interlobular septal thickening with predominant peripheral distribution.
MATERIAL AND METHODS
This observational study was conducted in the department of Respiratory Medicine, in collaboration with the department of Radiodiagnosis, at King George’s Medical University, a tertiary care center in Northern India, from August 2020–January 2021 (total duration: 6 months), after obtaining clearance from the Institutional Ethics Committee.
Inclusion criteria was all consecutive patients aged ≥13 years who had moderate to severe COVID-19 infection that was serologically confirmed; had a CT thorax obtained at the time of diagnosis; had passed a duration of at least 3 months since the time of diagnosis; and provided written informed consent. The severity of COVID-19 was defined on the basis of Indian Council of Medical Research (ICMR) guidelines, according to which a respiratory rate ≥24 breaths/min or percentage saturation of oxygen ≤93% on room air constituted moderate disease, while a respiratory rate of >30 breaths/min or percentage saturation of oxygen <90% was needed to categorise the disease as severe.19 Those individuals who had their baseline CT thorax carried out at other centres but had their investigation records were allowed. The range of duration included since the time of diagnosis was between 94±4 days. Individuals who failed to provide consent, or had pre-existing respiratory comorbidity, were excluded.
Statistical Analysis
The statistical analysis was done using Statistical Package for Social Sciences (SPSS) version 21.0 (IBM, Armonk, New York, USA). The data was expressed as mean, median, standard deviation, or percentage, as was appropriate. All the categorical data were compared using a chi-squared test. Continuous variables in two groups were compared by t-test. Pearson’s correlation was used for correlation. Binary multivariate logistic regression analysis was performed in each patient to determine the independent risk factors for severity of COVID-19. The p-value <0.05 was considered as significant.
Participants and CT Scoring
Eighty-nine patients who presented to the outpatient department with persistent symptoms 3 months after suffering from COVID-19 were screened for the study. A detailed clinical history, including the history of treatment received and past history, were taken. After applying the inclusion and exclusion criteria, 56 subjects were included in the study. All were subjected to high resolution CT (HRCT) thorax in supine position (standard-of-care) using 120 kVp/80 mAs with scan time of 1 sec with slice thickness of 1 mm, using the same machine and settings. The lungs were evaluated at window width of 1,000–1,500 HU and window level of -550 to -650 by the same radiologist and findings noted.
CSS was calculated by assigning each of the five lung lobes a score from 0–5 based on percentage involved.18 Score 0 implied no involvement; 1 was allotted if there was <5% involvement; 2 if 5–25% was involved; 3 for 25–50%; 4 in cases of 50–75% involvement; and the maximal lobar score of 5 for >75% involvement. The individual lobar scores were added to arrive at the final score ranging from 0–25.
RESULTS
A total of 56 subjects were recruited, out of which 32 (57.1%) were males. The mean age of the study population was 40.1 years. Other baseline characteristics are compiled in Table 1. Out of 56 subjects, 15 had suffered from moderate COVID-19, while 41 had had severe disease. Smoking history was comparable among the subjects. Diabetes was the most frequent comorbidity and pneumothorax the most common associated complication. The mean age was 45.67±14.50 and 38.05±11.56 years, respectively, for patients with and without comorbidities (difference: -7.61; [-14.82; -0.41]; p=0.043).
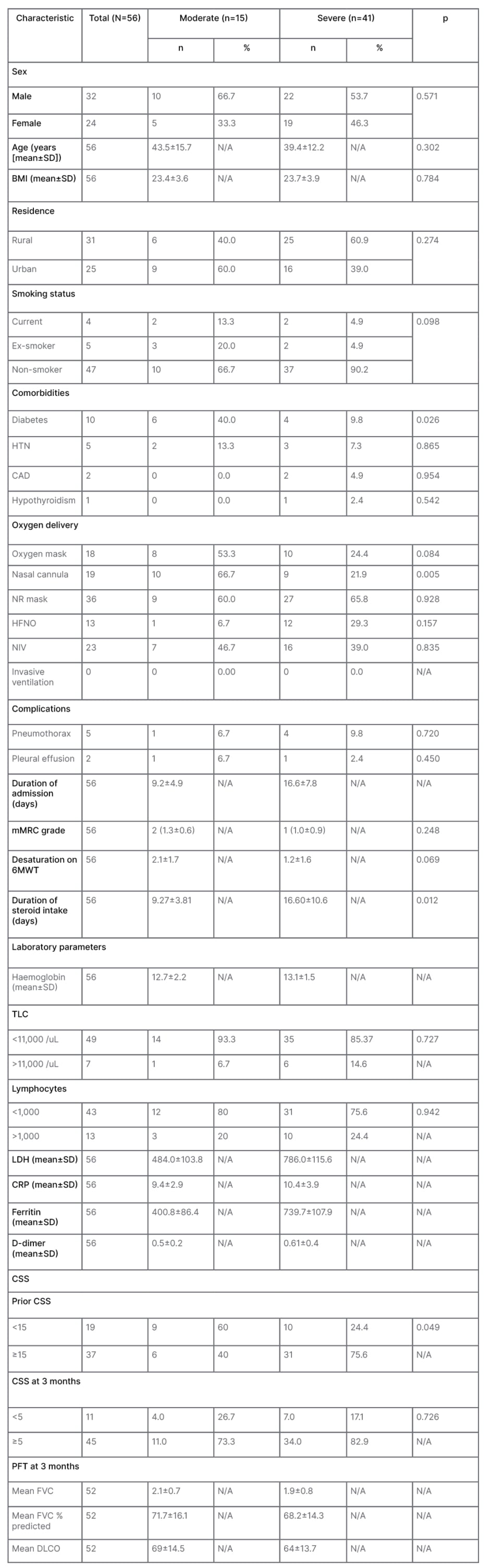
Table 1: Baseline data of the study population.
CAD: coronary artery disease; CRP: C-reactive protein ; CSS: CT severity score; DLCO: diffusion capacity of the lungs for carbon monoxide; FVC: forced vital capacity; HFNO: high flow nasal oxygen; HTN: hypertension; LDH: lactate dehydrogenase; mMRC: modified Medical Research Council; N/A: not applicable; NIV: non-invasive ventilation; NR: non-rebreathing; PFT: pulmonary function test; SD: standard deviation; TLC: total leukocyte count; 6MWT: 6-minute walk test.
The mean duration of hospital stay for moderate cases was 9.20 days, while that for severe cases was 16.56 days. None of the subjects had undergone invasive ventilation. Median CSS at the time of admission among moderate and severe cases were 16 and 17, which reduced to five and six, respectively, by the end of 3 months. At the time of diagnosis most patients had total leukocyte count within the normal range with lymphopenia (Table 1 and Figure 1). Only 52 subjects out of 56 were able to perform acceptable pulmonary function test. The mean values fell within the range of mild restriction with mild diffusion defect. The history of corticosteroid intake was significantly higher among the severe group. A significant positive correlation (Karl Pearson’s correlation coefficient 0.302; p=0.024) was seen between the CSS and duration of steroid intake.
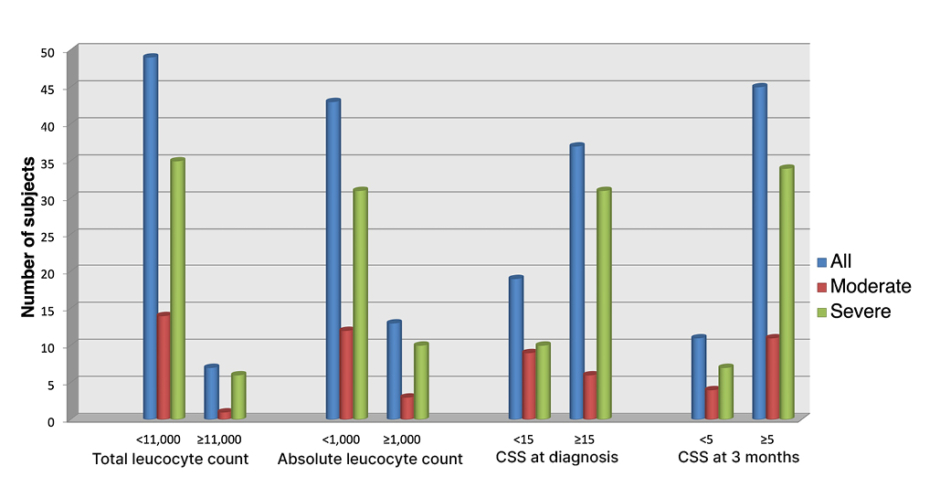
Figure 1: Graph depicting most of the subjects having normal leukocyte count, low lymphocytes, a CT-
severity score of >5 at 3 months and >15 at the time of diagnosis.
CSS: CT-severity score.
The most common complaint was persistent fatigue in over 90% subjects of both groups, followed by cough (32.1%) and dyspnoea (28.6%). Diarrhoea persisted in only two subjects of severe COVID-19 (Table 2).
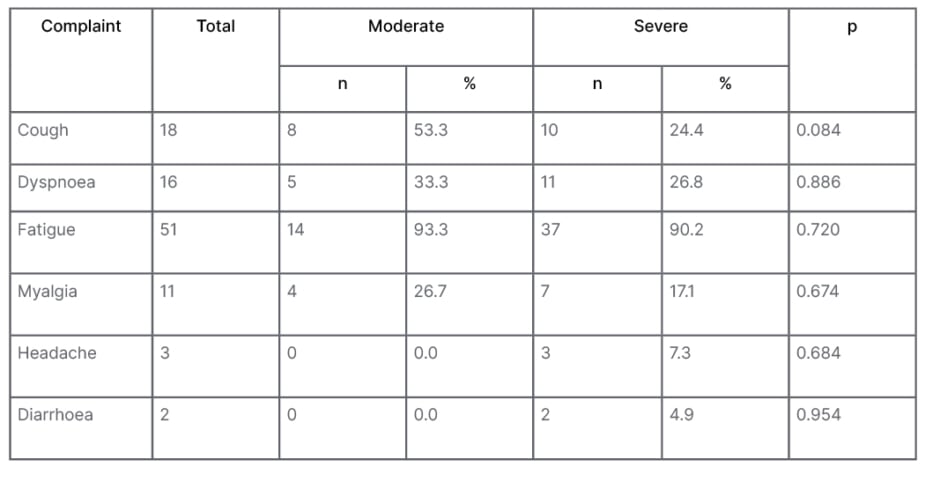
Table 2: Comparisons of persistent complains after 3 months in between patients with moderate and severe COVID-19.
The radiological findings persisted more in severe COVID-19 subjects. Bilateral involvement was seen in 89.3% (n=50) with peripheral distribution in 69.6%. Lower lobes were most frequently involved in the severe group. The most common residual radiological abnormality was patchy ground glass opacity that was seen in 89% patients; the next most common finding was reticulation (86%) with a specific pattern of distribution in the subpleural region (Figure 2). Crazy paving, pneumothorax, and pleural effusion were the least common findings. Other features are tabulated in Table 3. Few interesting cases are depicted in Figure 3.
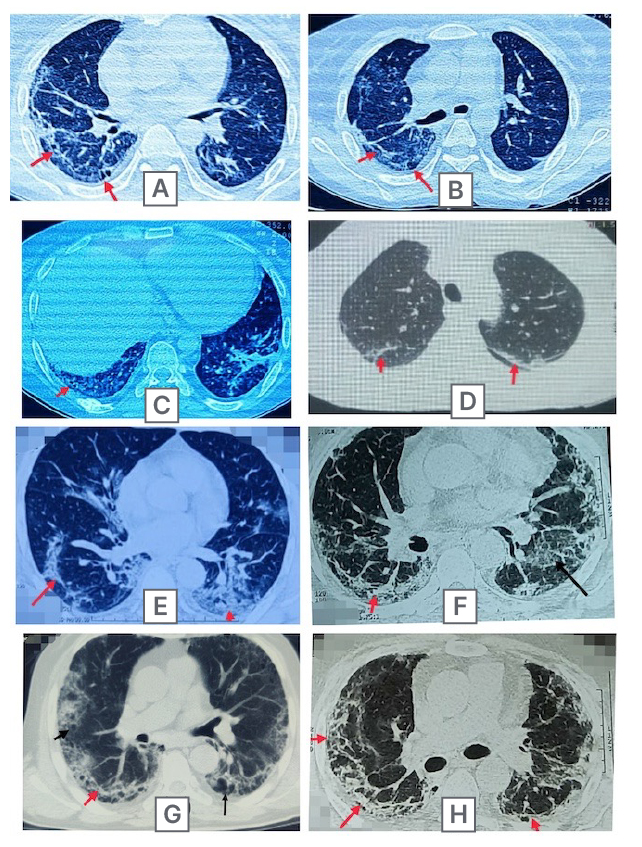
Figure 2: Three month follow up high resolution CT thorax images of multiple post-COVID-19 subjects demonstrating the curvilinear subpleural fibrotic band (red arrows) with development of cystic changes (black arrows).
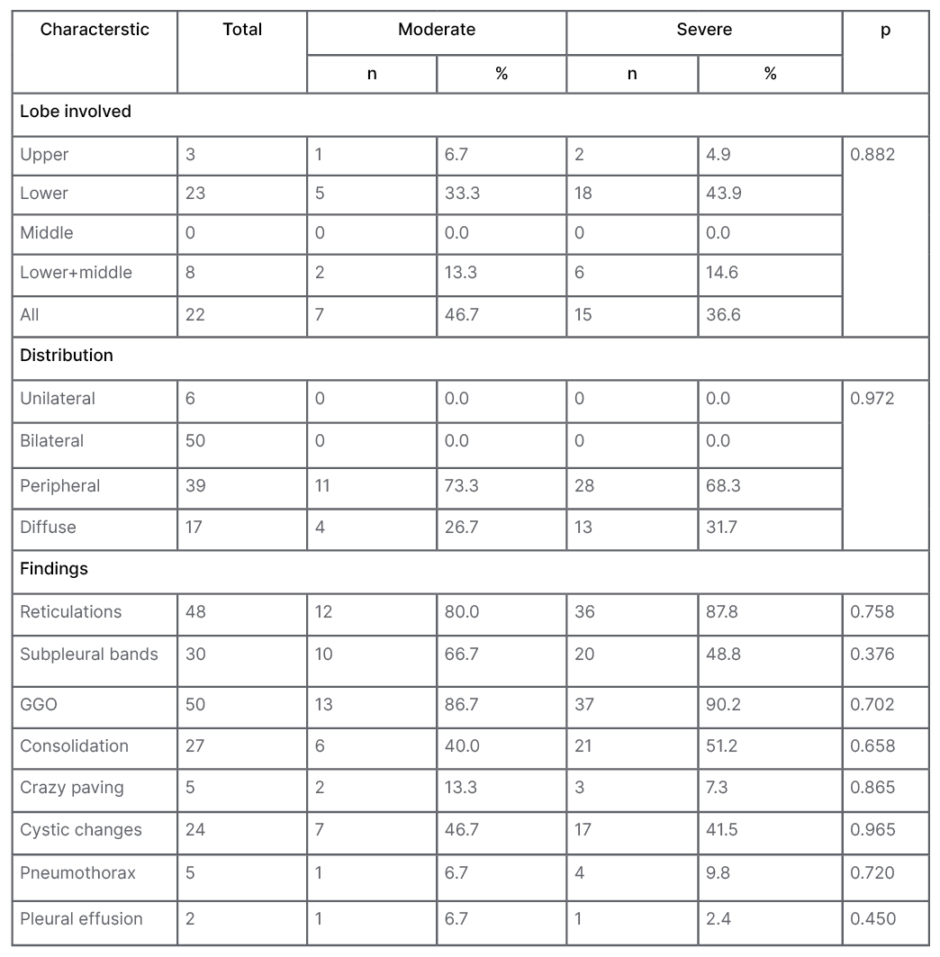
Table 3: Comparisons of persistent radiological findings after 3 months in between patients with moderate and severe COVID-19.
GGO: ground glass opacity.
On bivariate analysis, the mean age was 45.67 years; this was significantly higher (p=0.043) in subjects with coexistent comorbidity than those without it (mean age: 38.05 years). The mean age was 41.87±13.83 and 38.92±11.95 years, respectively, for patients with and without subpleural bands (difference: 2.94 [-4.04; 9.92]; p=0.41). The authors could not find any significant association between dyspnoea and CSS (Karl Pearson’s correlation coefficient 0.171; p=0.166). In multivariate analysis, no correlation could be found between age, BMI, duration of hospital stay, comorbidities, and persistent subpleural band at the end of 3 months.
DISCUSSION
In this cross-sectional study, the authors aimed to study the different radiological findings in post-COVID pulmonary fibrosis. Fifty-six subjects were recruited, excluding the paediatric population. The most common presenting complaints were fatigue, dry cough, and shortness of breath on exertion. The HRCT findings were mostly bilateral with no sex predilection. Ground glassing continued to persist in most cases, followed by reticular markings. The curvilinear fibrosis parallel to pleural surface with radiating thin fibrotic bands to the pleura, as have been defined in CO-RADS 5 staging, was a classic finding.17
While most studies included patients in either diseased or early recovery phase, the authors recruited patients after a period of at least 3 months to assess which abnormalities continue to persevere. The authors’ results correspond to the aftermath of the first wave of COVID-19 in India. Shi H et al.2 have reported similar prevalence of ground glassing and fibrotic changes. Nabahati et al.7 reported septal thickening in 83.3% and parenchymal bands in 64.4% of their subjects at the end of 3 months, similarly to this study. Ali and Ghonimy8 reported residual fibrosis in nearly one-third of their subjects; this was lower than in the authors’ study. Ali and Ghonimy8 had excluded subjects with chronic comorbidities, which might be responsible for a lower percentage of residual abnormalities, along with geographical variation in management facilities and protocols.
Initially, the management of COVID-19 revolved around antiviral and immune-modulatory drugs only, along with supportive care.20-23 The turnaround point was the RECOVERY trial24 that reported mortality benefit with the use of corticosteroids; since then, these have been part of treatment protocols all across the world in moderate to severe disease.25 The authors’ subjects too had received glucocorticoids for an average duration of 14 days, and the duration of usage was significantly higher in the severe group. Despite that, a fairly high percentage had abnormal parenchyma on CT thorax. Even for the treatment of post-COVID-19 pulmonary fibrosis, corticosteroids have been utilised and have been associated with improved clinical and radiological outcome.26
Linear parenchymal bands have been long associated with asbestosis, representing septal fibrosis along with distortion of the architecture.27,28 Less classically, linear fibrotic subpleural opacities have been seen in sarcoidosis, ankylosing spondylitis, systemic lupus erythematosus, etc.29-31
These disease conditions are all associated with chronic ongoing inflammation. Among acute conditions, in some survivors of acute respiratory distress syndrome, Middle East respiratory syndrome, and SARS, fibrotic sequelae have been reported in the lungs.32-34 Presence of
these bands early in the course of COVID-19 were also described as predictor of pulmonary fibrosis by Yu et al.35 The pathophysiology responsible for such rapid development of fibrosis in the lungs is believed to be over-triggered host immune response, along with the accompanied cytokine storm of molecules such as tumour growth factor β.36
It is worth remarking that the authors included only those subjects who presented with persistent symptoms, while several others followed up their cohort of consecutive COVID-19 patients, irrespective of symptom status, and yet the persistence of HRCT findings is similar. This suggests even asymptomatic subjects may be having parenchymal abnormalities, as ‘happy hypoxia’ was a common phenomenon during the first wave of COVID-19.37,38 However, this sample size is too small for a conclusive comment.
Remnant parenchymal changes were also seen in SARS and Middle East respiratory syndrome, and were seen to regress with time.39 Nabahati et al.7 reported diminution of fibrosis in nearly one-third of their subjects in the 6-month follow-up. More long-term follow-up studies with larger sample sizes will further help to assess the course of such fibrotic changes.
This study has the limitations of recruiting only moderate to severe cases, and not following the entire cohort. The authors could not include mild cases, as most patients attending their department with residual symptoms had suffered from moderate and severe disease. The small size of only 56 subjects is certainly a drawback in drawing significant conclusions.
CONCLUSION
Whenever the differential of fibrotic lung diseases will be considered, post-COVID-19 pulmonary fibrosis also needs to be discussed as a potential aetiology. The prominent patterns include ground glassing, septal thickening, and patchy consolidaton. Subpleural arcade of fibrosis was a peculiar feature.








