Abstract
A physically active lifestyle has health benefits, including enhanced vaccination responses, improved neutrophil and macrophage function, increased T cell proliferative capacity, lower numbers of senescent T cells, and lower levels of inflammatory cytokines. Therefore, exercise or physical activity is effective for preventing and treating chronic diseases. A more robust immune response is generally thought to be exerted in females than males in response to various challenges. Sex hormones in both sexes have been suggested as mediators of immune function, but research on this topic has not been designed with a sex-specific lens. The authors reviewed and summarized the experimental and clinical evidence in the available literature linking exercise, immune function, and risk of upper respiratory infections, as well as associated mechanisms. Collectively, the available literature indicates that moderate exercise improves immune function and risk for upper respiratory infections in both sexes. In contrast, prolonged and high-intensity exercise temporarily impairs immune responses and upper respiratory infection risk at a higher degree in females than males. Therefore, moderate exercise and activity may enhance immune function regardless of sex, whereas prolonged and high-intensity exercise temporarily impairs immune responses, predominantly in females more than their male counterparts.
Key Points
1. For far too long, the study of exercise and its effects on the immune system has overlooked a crucial factor: sex. This article delves into this critical gap by investigating sex-specific responses to exercise in the context of respiratory infections and immune function, a topic with far-reaching implications for public health, and personalized exercise guidelines.
2. This article reviews experimental and clinical evidence available in the literature, revealing how exercise interacts with the immune response to infection in a sex-specific manner.
3. Prolonged and high-intensity exercise temporarily impairs immune responses and increases the incidence of respiratory infections, with more predominant effects in female than male subjects. Therefore, sex-specific personalized interventions that optimize immune benefits, and minimize infection risk, should be implemented to improve public health outcomes.
INTRODUCTION
Physical activity is an efficient drug-free strategy which has been associated with multiple health benefits, including mental and physical health, in subjects from both sexes.1 More than 100 years ago, Larrabee2 observed a marked leukocytosis after violent exercise, including many polymorphonuclear neutrophils. Since then, research on exercise-induced effects on the immune system has advanced tremendously, and reports about positive effects on health, particularly in delaying disease-related mortality, have flourished.3 Studies conducted in patients of all ages, but particularly middle-aged and older adults, have consistently shown that physical activity delays cellular aging (e.g., telomere length, immunosenescence), and reduces the risk of metabolic, respiratory, and cardiovascular disease.4,5 Overall, the practice of regular exercise has been associated with changes in immune profiles, resulting in lower inflammatory states, improved mitochondrial function, and enhanced innate and adaptive immune responses.6,7
Many factors in males and females, including sex hormones and their receptors, regulate immune system function, immune cell profiles, and response to immune challenges.8-13 Generally speaking, females have been reported to have stronger immune responses than males.14 Furthermore, female sex hormones such as estrogen, progesterone, and chorionic gonadotropin have been shown to modulate function, differentiation, and both cytokine and antibody production in various immune cells, including monocytes, dendritic cells, and lymphocytes, across the life span.15-18 On the other hand, dominant male gonadal hormones, such as testosterone, also play important roles in regulating immune responses.19
The available literature on this topic suggests that the immune system’s response to exercise strongly depends on the duration and intensity of the physical activity performed, and involves changes in multiple immune cells. This idea has taken root in the scientific literature, to an extent by which aerobic exercise, particularly moderate-intensity exercise, can be beneficial for the immune system, but vigorous and prolonged exercise, on the other hand, can interfere with immunocompetence. The current study explores these scenarios in the context of male- and female-specific responses.
Here, the authors focus on acute respiratory illness, which is one of the leading causes of global morbidity. Before the COVID-19 pandemic, the incidence of upper respiratory infections reached 17.2 billion people in 2019, accounting for 42.83% of cases from all causes globally. The average adult has approximately one to six colds yearly.20 Therefore, in this review, the authors aim to provide updated and concise information on the impact of exercise on respiratory infection as immune responses to moderate and vigorous exercise, with a lens to specific sex differences.
MATERIALS AND METHODS
Databases and Key Terms Searched
The authors used both Google Scholar and PubMed databases to identify eligible papers in their search. They used the following search terms and logic to select articles: physical activity OR exercise AND exercise-induced immune response AND immune response OR sex differences AND sex hormones OR male OR female.
Inclusion Criteria
The authors conducted the search without limit of the year of publication, limited to papers written in English. The final search was updated to November 2023. Articles were selected if they assessed the effects of moderate-intensity exercise (30–45 min/day) or vigorous exercise (endurance-trained athletes) on the immune system, through the incidence of acute respiratory illness, including study populations of 18–65 years old of both sexes. Upper respiratory tract infection (URTI) incidence was assessed by self-reporting (using health logs), or diagnosis reported by a physician.
Search Process and Study Selection
All articles were selected based on the search criteria. Articles were de-duplicated using built-in mechanisms of the authors’ university’s library services, and assessed by their titles and abstracts for inclusion. Final selections were determined after thoroughly reading the articles’ methodological design, methods, risk for bias, and data analysis, to meet the authors’ inclusion criteria and answer their research questions, as well as by the ability of the results presented to be generalized for all selected studies. To minimize bias, all articles were reviewed independently by each author following the inclusion and exclusion criteria explicitly, which were established prior to the search process and study selection.
Data Extraction and Organization
The information extracted from the studies was as follows: the first author of the study, the year of publication, the study design and/or details of the intervention, the sample size (number and/or percentage of population), and the study’s critical outcomes. Upon review of the information presented, articles were categorized and organized in tables according to the reported response to moderate-intensity or vigorous exercise. No statistical analyses were conducted as part of this process.
RESULTS
Search Results
A total of 984 studies (Figure 1) were identified using the aforementioned key terms searched, of which 132 were duplicates. Of the remaining 852 studies, 759 had irrelevant study design or settings, or did not explicitly define the sample size or interventions, and were excluded. The full text of the remaining 93 articles was reviewed. The final selection, excluding studies that did not meet the inclusion criteria, comprised 32 articles. These were subsequently divided into articles studying moderate-intensity exercise (10 articles, listed in Supplementary Table 1) versus vigorous exercise (23 articles, listed in Supplementary Table 2).
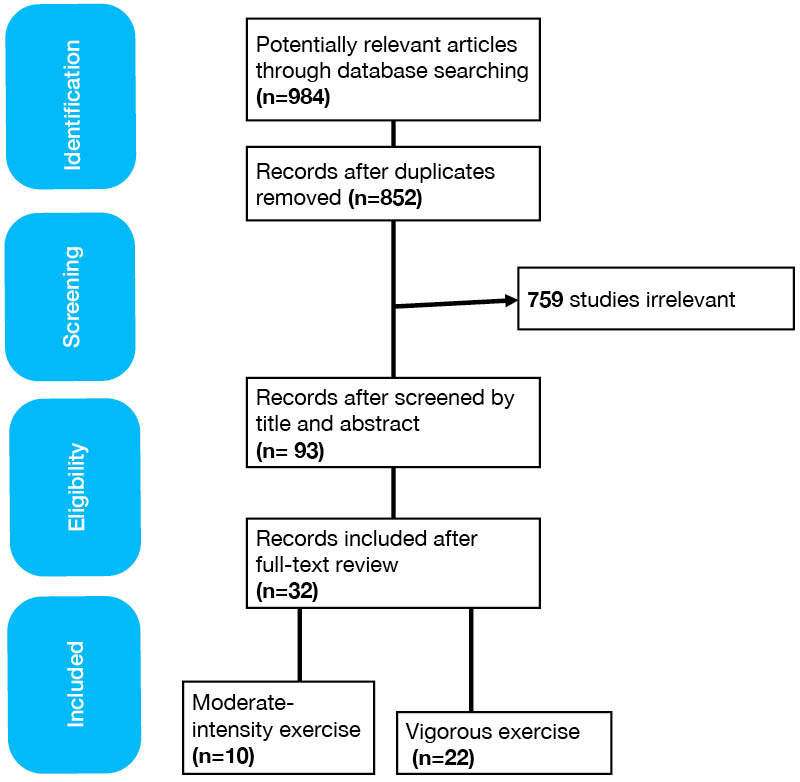
Sex differences in exercise-induced effects on respiratory infection
Effects of Moderate Physical Activity Intensity on Immune Responses in Female and Male Subjects
The literature review identified 10 publications linking moderate-intensity exercise with immune response and URTI outcomes. The moderate-intensity exercise studies consisted of brisk walking, stretching, jogging, or mild aerobic exercise, and the main results, study design, participant information, and study goals are summarized in Supplementary Table 1. The authors identified four studies including only female participants,21-24 six papers that included both male and female subjects,25-30 and zero studies that included only males. Overall, enrolment of subjects ranged from 32–1,509 for the selected studies. The combined total of participants from all studies was 5,284 (including 3,442 females and 1,842 males), and 0.93% (n=32) to 23% (n=791) were female participants. Interestingly, in all studies enrolling only females, it was reported that moderate physical activity was associated with lower rates of URTI. Also, in all four studies that included females, subjects were sedentary, overweight, or obese. Only one study that compared the effect in highly conditioned females found a reduced effect in this group versus the sedentary group.22
On the other hand, the studies that enrolled participants from both sexes found that moderate-intensity physical activity was associated with solid reductions in URTIs, and fewer symptomatic days when compared with subjects that were sedentary. Unfortunately, while these studies included subjects of both sexes, comparisons between effects in males and females were not provided, except for one study that showed a more substantial effect in females than males in reducing URTIs.28
Effects of Vigorous Physical Activity Intensity on Immune Responses in Females and Males
The literature review identified 22 publications linking vigorous exercise (e.g., long-distance running, swimming, endurance training, triathlon, track and field, athletics, Olympic sports, and cross-country skiing) and immune-related outcomes (Supplementary Table 2). Of these, five studies did not report the sex of the participants,31-35 whereas the remaining 17 studies enrolled both sexes.36-45 These studies enrolled a range of participants, from as low as 20 to as much as 11,274 subjects, of whom 0.12–26.00% were female. Interestingly, participants engaging in vigorous exercise displayed an increased incidence of illness compared to controls. For example, during periods of heavy training, or after a race, marathon runners had higher rates of infectious episodes. In these associations, female sex was recognized as a risk factor for athletes, as illness incidence was higher in females in nine studies that compared effects in both sexes.39,42-49
DISCUSSION
An extensive volume of peer-reviewed studies in exercise immunology has established links between immune function and exercise.50 High levels of physical activity have sometimes been associated with decreased circulating sex hormones in pre- and postmenopausal females, and alterations in susceptibility to infection and disease.51,52 Hence, it is evident that biological sex plays a vital role as an essential immune response modulator. Sex hormones, including estrogens, can act as enhancers of humoral immunity, and androgens and progesterone (and glucocorticoids) can act as natural immunosuppressants.53 Therefore, it was not surprising to find that multiple studies had identified sex differences in immune responses in both exercising and non-exercising conditions.54,55
In the current study, the authors sought to identify sex differences in the effects that various exercise modalities exert on immune responses, particularly on the association of URTI incidence, by reviewing the available literature in the topic. Overall, the authors confirmed that multiple studies conducted in the field are not designed or powered to test for sex as a biological variable, nor attempted to evaluate the contributions of sex hormones to measured outcomes. However, the authors’ literature search identified many effects of exercise on immune function that appear to correlate with the intensity of the bouts of exercise, and modulated by sex.
The search revealed that, regardless of sex, individuals engaging in moderate-intensity exercise displayed fewer episodes of URTIs, and experienced fewer days with symptoms when compared to subjects who were sedentary. Moderate-intensity exercise was associated with an improvement in immune function in individuals who were previously sedentary. It is important to note that most of the studies reviewed here did not account for the sex variable in the design, analysis, or data reporting. Only one study indicated a higher incidence of URTIs in female subjects, when compared to males, although this effect was mild.28 Together, the available literature supports the notion that moderate exercise is beneficial to prevent infection and improve immunity in both male and female individuals.
In contrast, when reviewing the literature on vigorous exercise and its effects on immune function, and incidence of URTIs, the authors repetitively found that different forms of intense and vigorous exercise were reported to exert a negative effect on the immune system’s phenotypic makeup and functional capacity. In most cases, it was found that both URTIs and symptomatic days were higher in individuals involved in vigorous exercise, as shown in Supplementary Table 2. Interestingly, studies incorporating both male and female individuals reported that females displayed a higher incidence of illness in most studies, indicating that female sex represents an additional risk factor for the negative effect of intense exercise. Despite these findings, most studies were not designed to detect sex-specific effects, or disaggregate all data by sex. Thus, it is essential to emphasize that future work should follow the guidelines for Sex and Gender Equity in Research (SAGER) in the design, analysis, and reporting of findings.56
In sum, the available literature in this topic indicates that both the duration and intensity of exercise may affect the immune system’s response to infection, and that these effects may be influenced by fitness level and circulating sex hormone levels. This work suggests a modulatory crosstalk of female sex hormones and exercise-induced physiological responses. However, many of these studies have significant limitations, because both sex differences and the role of sex hormones were not elaborated on, or investigated, or because the geographic scope of participants differed across studies, and was more global in studies assessing vigorous exercise than moderate exercise. Despite this, the present study has solid implications for public health, as athletes, who are often considered healthier than the average population, are more prone to infection due to immune system alterations that occur during, and after, periods of heavy training. The current literature review confirms this, and indicates that female sex represents a risk factor in multiple exercise modalities and populations. Hence, medical personnel, coaches, and athletes must be aware of the increased risk of illness during vigorous exercise, particularly in females.
While the current study provides a comprehensive assessment of the relationship between exercise and immunity, it also has several limitations. First, while the reports reviewed were conducted in different countries, most studies investigating moderate exercise effects on immune function were conducted in the USA. In contrast, studies investigating vigorous exercise were conducted in multiple countries. Second, this study focuses on studies addressing the effects of URTIs in professional athletes, which may limit its generalizability to other populations. Finally, information about participants’ BMI, medication use, and other medical conditions was only sometimes available in the studies consulted. This limited the authors’ ability to assess their impact on the effects reported in males and females. Clinically, this manuscript is relevant, as it confirms that there is solid evidence for the effectiveness of moderate physical activity in supporting the immune system, especially in previously sedentary people, but also found an increased risk of URTIs in females compared to males, in those who partake in vigorous exercise. Therefore, clinicians who care for females need to be aware of these findings to develop individualized exercise training programs. The authors also encourage further research on the underlying factors and potential roles of sex hormones in immunity and respiratory infection risk.
Overall, the body of literature summarized in the current study suggests that promoting training programs with increased low-to-moderate intensity training volumes, and ensuring sufficient rest and recovery, may help maintain immune health, and decrease the incidence of infection. More research must be done to fully elucidate the contributions of sex-specific factors to these outcomes, and the mechanisms underlying sex-specific immune responses to exercise. In conclusion, the available literature suggests that moderate exercise may enhance immune function, regardless of sex. In contrast, both prolonged and high-intensity exercise regimes are associated with temporary impairment of immune responses, with more predominant effects in female than male subjects.







