Meeting Summary
The objective of this symposium was to build on the guiding principles of asthma and chronic obstructive pulmonary disease (COPD) and to address some of the most frequently encountered challenges in the management of chronic airway disease, using a mix of scientific information and guidance based on clinical practice. Prof Mäkelä opened the symposium by reviewing key achievements from the Finnish asthma, COPD, and allergy programmes. He also highlighted how these co-ordinated educational programmes were responsible for driving an improvement in Finnish public health and reducing the socioeconomic burden of disease. Prof Chrystyn then addressed some of the common misconceptions associated with high-resistance dry powder inhalers; he explained how the properties of these devices make them suitable for use by a broader range of patients than perceived by many clinicians. Next, Prof Lavorini addressed the real-world use of inhalers by highlighting how specific errors in recent real-life studies are associated with a loss of disease control and how the Easyhaler® (Orion Corporation, Espoo, Finland) meets many of the needs of doctors and patients. Finally, Prof Canonica focussed on precision and personalised medicine in chronic airway disease, with an emphasis on how clinicians can optimise patient adherence and, consequently, treatment in daily practice.
Transforming Asthma and COPD Care: Lessons from Finland
Professor Mika Mäkelä
Over the past few decades, Finland has experienced measurable successes in improving asthma and COPD care, largely due to nationwide and co-ordinated programmes designed to educate healthcare professionals (with a strong emphasis on primary care) and inform the general public.
Early History
The first of these programmes, the 10-year asthma programme, commenced in 1994. It was designed to lessen the burden of asthma on individuals and society through early diagnosis and treatment (with guided self-management as the primary form of treatment); reduction in respiratory irritants, such as smoking; implementation of patient education and rehabilitation combined with normal treatment; an increase in knowledge about asthma in key groups; and promotion of scientific research.1 Overall, the programme had a considerable impact on healthcare use and disability. By 2003, it reduced the number of hospital days attributable to asthma compared with those in 1981.1 The total cost of asthma also reduced, from €222 million in 1987 to €191 million in 2013 (a reduction of 14%), despite a three-fold increase in the number of asthmatics from 83,000 to 247,583.2 The success of the programme has been largely attributed to education, which was central in helping to identify and treat patients and improve guided self-management.
Improvements in COPD Care
The Finnish COPD programme overlapped with the asthma programme and was equally successful in bringing about much needed improvements to COPD care. Using a multilayered approach, this programme was designed to reduce the prevalence of COPD; improve COPD diagnoses (especially in primary care); and reduce the number of moderate-to-severe cases of COPD, hospitalisations, and treatment costs due to COPD.3 Programme implementation included the provision of information for healthcare professionals and the general population, as well as multidisciplinary education and training. In parallel, the publication of guideline statements and the introduction of tobacco legislation over the same timeframe supported the programme’s agenda and helped to ensure its success. In terms of outcomes, the prevalence of COPD remained unchanged over the programme’s course, but it was successful on multiple other fronts. For example, smoking decreased in men and women, significant improvements were observed in the delivery of spirometry, and there was a statistically significant reduction in costly hospitalisations (39.7%) between 1997 and 2007.3
Targets for the Future
Most recently, the Finnish allergy programme was introduced to address the growing epidemic of allergy. Capitalising on the successes of the asthma and COPD programmes, it has set out a number of ambitious targets, including a reduction in allergies by 20%, work-related allergies by 50%, avoidance diets by 50%, emergency visits caused by asthma by 40%, and allergic disease-related costs by 20%, as well as the availability and use of quality-certified allergy testing centres for all patients.4 However, rather than seeking to drive behaviour change among healthcare professionals, the allergy programme has targeted patients with allergies and asthma and their families, public health and patient organisations, experts, legislators, and authorities. Full results from the programme, which is due to conclude in 2018, will be disclosed in due course, but results from the first 8 years have been promising, with improvements reported across all but one indicator.
Overall, the Finnish asthma, COPD, and allergy programmes have shown how the burden of chronic respiratory diseases can be reduced with national consensus and the implementation of regional education and disease awareness campaigns. While sound clinical science is needed as a fundamental basis for education, common sense and the provision of practical information are also needed in equal measure to bring about meaningful change and an improvement in public health.
Making Sense of Dry Powder Inhalers
Professor Henry Chrystyn
Dry powder inhalers rely on the generation of a turbulent airflow energy to sufficiently break-up (known as deaggregation) drug particles from their carrier (typically lactose) within the inhaler formulation. In its simplest terms, this turbulent airflow energy is the product of a patient’s inhalation flow and the resistance of a given inhaler.5,6 Therefore, to generate a set turbulent airflow energy through a dry powder inhaler, either a low inhalation flow through a high-resistance inhaler or a high inhalation flow through a low-resistance inhaler is required.5
Turbulent Airflow Versus Peak Inhalation Flow
Peak inhalation flows are lowest through high-resistance dry powder inhalers and highest through low-resistance dry powder inhalers in adults with asthma or COPD, and children with asthma.7 The opposite is true for turbulent airflow energy, which increases with dry powder inhaler resistance across all patient groups.7 It is this turbulent airflow energy generated inside an inhaler that is of greater importance than a patient’s peak inhalation flow for the deaggregation of the drug particles from their carrier during the operation of a dry powder inhaler.
The Importance of Inhaler Training
Across the spectrum of dry powder inhalers, only a minority of patients with asthma (adults and children) and COPD are unable to generate peak inhalation flows ≥30 L/min (Aerolizer® [Novartis Pharma AG, Basel, Switzerland]: 1.0%; Diskus® [GlaxoSmithKline, Brentford, UK]: 6.1%; Turbuhaler® [AstraZeneca AB, Södertälje, Sweden]: 4.1%; Spiromax® [Teva Pharma BV, Haarlem, Netherlands]: 1.3%; and Easyhaler: 3.1%),7,8 which is considered the effective minimum flow rate for dry powder inhalers. Given that most patients can achieve the threshold peak inhalation flow required for correct dry powder inhaler operation, irrespective of device resistance and disease severity, the use of high-resistance dry powder inhalers need not be restricted in patients unable to generate high inhalation flows, such as those with COPD. Dry powder inhalers with higher resistance, such as the Easyhaler, Twisthaler® (Merck Sharp & Dohme Ltd., Hertfordshire, UK), or Handihaler® (Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, Connecticut, USA), require lower inhalation flows than dry powder inhalers with lower resistance to generate equivalent turbulent airflow energies for drug deaggregation through the device (Figure 1).9,10 With this in mind, clinicians should focus on the provision of adequate inhaler training for patients, rather than placing emphasis on achieving a specific inhalation flow.11 Figure 1 shows that the resistance of dry powder inhalers that are formulated with fixed-dose combinations of a long-acting β-agonist and corticosteroid are similar.
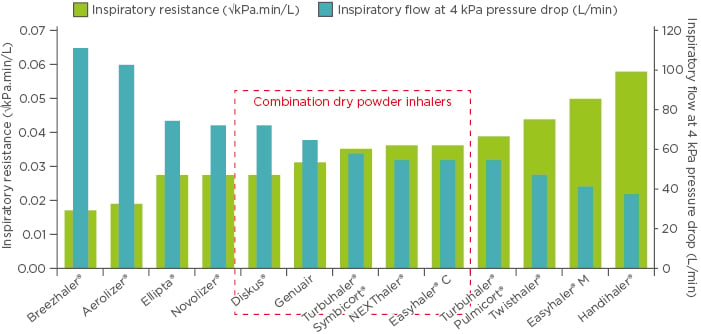
Figure 1: Inspiratory resistance of marketed dry powder inhalers and respective inspiratory flows studied at a pre-set equal pressure drop rate of 4 kPa.
Easyhaler® C: combination; Easyhaler® M: monotherapy.
Adapted from Malmberg et al.9 and Krüger et al.10
The inhalation parameters when patients use the Easyhaler make it suitable for all patients generating a range of peak inhalation flows. For example, studies have shown that peak inhalation flows and dose delivery with the Easyhaler are similar to another widely prescribed dry powder inhaler, the Turbuhaler,9 relative lung deposition with the Easyhaler is similar irrespective of the inhalation flow rate,9 and interpatient flow variability with the device is smaller to that of other dry powder inhalers.12 These attributes combine to provide consistent dose emission irrespective of the inhalation flow.12
Inhaler Use in Real Life: An Evolving Story
Professor Federico Lavorini
Randomised controlled trials are considered the gold standard of evidence for providing information on treatment efficacy. However, unlike real-life studies, which assess the effectiveness of a treatment under real-world conditions, they can seldom be generalised to a broader population.
Issues with Inhaler Use
According to one analysis, only 1.3% of patients in the real world meet the stringent eligibility criteria for clinical trials in asthma.13 Patient adherence is also frequently higher in randomised controlled trials than in real-life studies, in which it can fall below 50.0% and reach as low as 8.3%.14,15 There are many reasons why adherence may be suboptimal in the real world, including poor inhaler technique, incorrect inhaler use, and a lack of understanding of inhaler use.16
In a cross-sectional study of >3,500 patients with asthma (CRITIKAL),17 inhaler errors were found to be common and not exclusive to a specific type of inhaler (Figure 2). Specifically, insufficient inspiratory effort with both the Turbuhaler and Diskus inhalers was associated with an increased likelihood of uncontrolled asthma and exacerbation. By contrast, a lack of knowledge, incorrect preparation, incorrect timing of inhalation, incorrect head position, and hand–breath disco-ordination with pressurised metered-dose inhalers were associated with an increased likelihood of uncontrolled asthma but not exacerbation.17 Data from a recent real-life French study of almost 3,000 patients have indicated that inhaler handling errors are also frequent in patients with COPD (only 25.4% of whom did not make any error) and are associated with an increased rate of severe COPD exacerbations.18
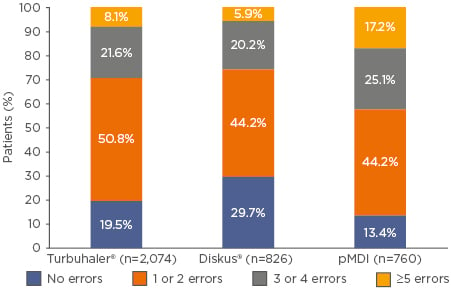
Figure 2: Number of errors made by patients in the CRITIKAL study, grouped by device type.
pMDI: pressurised metered-dose inhaler.
Adapted from Price et al.17
Taken in the context of a systematic review that found inhaler use has not improved among patients over the past 40 years (1975–2014),19 these results should collectively serve as an urgent call to action for clinicians to ensure that they include patient education as an essential component of disease management.
Overcoming Challenges
Given that inhaler errors are common in the real world and are associated with poor disease outcomes, it is important that clinicians select the right inhaler for each patient in real life. In practice, an ‘ideal’ inhaler does not exist. However, the Easyhaler meets a series of five specific criteria designed to accommodate the needs of technologists, doctors, and patients: effective, efficient, engaging, error-tolerant, and easy to teach.
Firstly, the Easyhaler can be considered an effective inhaler. It delivers particle sizes in the respirable range (1.9–4.9 µm) irrespective of inhalation volume or flow rate,20 and a real-life study has shown that a switch to the budesonide/formoterol Easyhaler significantly improved disease control after 12 weeks in patients with asthma and COPD.21 Secondly, the Easyhaler is efficient; it requires five essential steps for inhalation. Up to 78% of adult patients displayed correct use after the first visit in a real-life study of 1,016 patients with asthma or COPD in Hungary.22 Thirdly, the Easyhaler is engaging, with patients expressing a higher degree of satisfaction with the device (72.4%) compared with Turbuhaler (17.3%), pressurised metered-dose inhalers (12.7%), or Diskus (18.0%).21 Fourthly, the Easyhaler is error-tolerant, meaning that it delivers consistent dose emission irrespective of moisture, vibration, freeze/thaw conditions, and the effects of dropping.12 Finally, >90% of clinicians have described the Easyhaler as easy to teach, with 98.9% of patients learning the inhaler technique within 10 minutes (Table 1).21
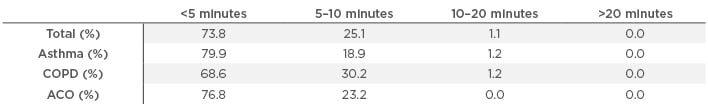
Table 1: Time taken to teach asthma, COPD, and ACO patients how to use the Easyhaler® (asthma: n=617; COPD: n=775; ACO: n=98).
ACO: Asthma–COPD overlap; COPD: chronic obstructive pulmonary disease.
Adapted from Tamasi et al.21
Focus on the Patient: What Clinicians Need to Know
Professor Giorgio Walter Canonica
The treatment of asthma and COPD is gradually evolving from a one-size-fits-all approach to one that encompasses both personalised and precision medicine. Although the terms personalised medicine and precision medicine have previously been used interchangeably, they should be considered separate concepts that have a collective role to play in disease management.
Defining Precision Medicine
Precision medicine can be defined as the use of targeted treatment based on the specific genetic, biomarker, phenotypic, or psychosocial characteristics of an individual patient. In other words, precision medicine targets a particular mechanism of disease.23,24 Over the years, technological advances in diagnostics and therapeutics have driven the evolution of precision medicine, supported by the growing field of omics, which includes proteomics, transcriptomics, and genomics. These have enabled clinicians to identify disease mechanisms using specific tools, subsequently allowing them to treat such mechanisms with highly targeted treatments, such as antibodies.
Personalised Medicine in Asthma and COPD
By contrast, personalised medicine focusses on the patient rather than the disease mechanism and accounts for the important role of patients in managing their own health. Choosing the correct inhaler for each patient is one aspect of personalised medicine that can have a tangible effect on adherence and, ultimately, control of chronic airway diseases. However, in reality, it is complicated by the fact that clinicians must choose from hundreds of possible inhalation products.25 It is, therefore, not surprising that patients find the use of multiple inhalers confusing26 and struggle to use their inhalers properly.27 In addition, switching from one inhaler to another has been shown to be problematic for patients. One retrospective matched cohort study of 824 patients from the UK found that patients with asthma whose inhaled corticosteroid was switched without an accompanying consultation experienced poorer asthma outcomes compared with controls.28
Other challenges to adherence include a lack of patient engagement. Results from the Global Asthma Physician and Patient (GAPP) survey29 have demonstrated how clinicians and patients have differing perceptions of education provided and received, with 64% of patients and 87% of clinicians reporting that up to half of office visits were devoted to educational issues.
Embracing Personalised Medicine
From a practical perspective, clinicians can incorporate a number of measures into their practice to improve inhaler use in patients switching devices and address the need to improve patient engagement. Firstly, education should be prioritised during clinician–patient consultations and clinicians should explain to patients why they are receiving a new inhaler and provide adequate instructions on how to use it correctly. Clinicians should also factor in frequent assessments of inhaler technique.30
These measures would align with patient expectations. A pragmatic survey consisting of face-to-face interviews conducted with a group of >2,000 individuals in Italy revealed that most patients want to be informed about how an inhaler works if they have to change device (86%), would rather maintain the same type of device (81%), and would be willing to change inhaler if their clinician told them to do so (85%).31
Overall, nonadherence represents a major barrier to the success of therapy but also an opportunity to improve health and wellbeing in patients with chronic airway diseases. Through better patient engagement, clinicians can help to improve adherence and deliver medicine that is truly personalised and focussed on the patient.
Summary
Professor Mika Mäkelä
Disease control in asthma and COPD is achievable in most patients using the inhalers and tools currently at clinicians’ disposal. Rather than abandoning the use of inhalers and medications that are supported by sound clinical science and have been successfully used for years, clinicians should reflect on their own practice and ensure that they are performing the basics correctly to promote optimal adherence to therapy. Crucially, patients should be supported in their efforts to remain adherent, and one effective way of doing so is through patient engagement with robust education and training.
Click below to view the following symposium videos:







