Meeting Summary
Prof Agustí opened the session by explaining the new challenges in airway diseases including the changing paradigm of our understanding of chronic obstructive pulmonary disease (COPD) that considers the entire lung function trajectory from birth to death, the complexity and heterogeneity of the disease, and the need to diagnose and treat COPD earlier in life. Prof Siddiqui then explained that all of the airways, including small airways, are critically important in the pathophysiology of asthma and COPD. The world’s largest multi-centre ATLANTIS study focussed on small airways dysfunction (SAD) confirmed that a simple combination of different assessments like oscillometry and spirometry could identify patients with the SAD phenotype. The prevalence of airway dysfunction in the full asthma cohort was 91%. Prof Papi discussed that exacerbations are a crucial event in the natural history of COPD and that they drive several health-related outcomes. He reviewed the clinical evidence to demonstrate the benefits of triple therapy in general and specifically of the extrafine fixed triple combination (beclometasone dipropionate, formoterol fumarate, glycopyrronium bromide) to consistently reduce the risk of exacerbations, and improve lung function and quality of life (QoL) with a favourable benefit-to-harm ratio. Furthermore, triple therapy showed promising signals in terms of improved survival. Prof Celli debated that inhaled corticosteroid (ICS) should be given to many patients because scientific trials have shown that: 1) ICS combined with bronchodilator (BD) are effective in improving health status and reducing exacerbations; 2) they also impact lung function decline and mortality; 3) ICS increase pneumonia risk (depending on type, dose, airflow limitation, BMI, and age) but have no untoward effect on mortality or hospitalisations for pneumonia; 4) blood eosinophil count (BEC) (<100 cell/µL) helps select patients unlikely to respond to ICS; and 5) ‘many’ COPD patients benefit from ICS combined with BD. Prof Singh focused on the fact that the magnitude of clinical benefit in preventing COPD exacerbations varies between individual patients, underlining the importance for clinicians of making the right decision for each patient when prescribing ICS, by balancing the potential risk/benefit. He concluded the debate by outlining that ICS have benefits in patients at increased exacerbation risk, and that the size of the benefits varies with BEC and the number/type of exacerbation.
![]()
Erratum: Building Hope by Restoring Breathing in Airways Diseases
Chairpeople: Àlvar Agustí
Speakers: Àlvar Agustí, Salman Siddiqui, Alberto Papi, Bartolome R Celli, Dave Singh
Original citation: EMJ Respir. 2019;7[1]:32-41.
Date correction published: 28.11.19
The article by Àlvar Agustí et al. in the November 2019 issue of EMJ Respiratory 7.1 (pages 32-41) was originally published on 14.11.19. Since then an erratum has been made. In Figure 1, the top graph line starts at 0 on the Y axis it originally started at 1 year, this has been updated. In Figure 2, the label “AX*” originally read “R5-R20*” incorrectly, this has now been updated.
The EMJ apologises for the error and any inconvenience caused.
![]()
New Challenges in Airway Diseases
Professor Àlvar Agustí
According to Prof Agustí, there are currently three key challenges to overcome to build hope in airway diseases. The first challenge is that the COPD paradigm is changing. Airway diseases (as many other chronic conditions of the adult individual) can start early in life (during pregnancy, infancy, and adolescence), albeit it is often not diagnosed until the sixth of seventh decade of age. Several lung function trajectories (Figure 1) exist throughout the life course, including a growth phase, a plateau phase, and a declining phase. This novel perspective provides a dynamic way to study how lifetime influences health and disease, including COPD. Therefore, time considerations in the understanding of airways disease are becoming increasingly important. Recent research has shown that 4–13% of the general population do not achieve normal peak lung function in early adulthood, albeit approximately two-thirds of children with reduced lung function at birth can catch up to a normal trajectory. At the other end of the spectrum, there are supranormal trajectories. These supranormal individuals may lose significant amounts of lung function through life and, nonetheless, present to the clinic in their sixties with evidence of lung damage (e.g., CT emphysema) and ‘pseudonormal’ spirometry.2,3Furthermore, low peak lung function in early adulthood is significantly associated with early development of comorbidities and premature mortality.3In total, there are two key biological phenomena (organ development and ageing) that need to be considered to better understand the pathogenesis of COPD.2,3In this context, COPD and asthma may be considered as a continuum of chronic airway diseases.1,4-9
The second challenge is that COPD in particular, and airway diseases in general, are complex and heterogeneous; therefore, one therapeutic strategy does not fit all patients. Disease complexity is defined by the presence or severity several components such as emphysema, exacerbations, rate of lung function decline, early life events, and inflammation, among others, and that these components are not linearly related (so one cannot be predicted from others). Furthermore, heterogeneous means that not all these components are always present in all patients or, in a single patient, they may change with time, either because of disease progression and/or the effect of therapy. To address these complexities and heterogeneity, it has been recently proposed that a strategy based on the presence of specific treatable traits may be the way forward for personalised and precise treatment of these patients.10-13This is supported by the recent Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendation to individualise treatment based on two key treatable traits, namely emphysema and dyspnoea.
The third and final challenge, following directly the realisation of different lung function trajectories leading to COPD (Figure 1), is to diagnose and treat the disease much earlier. Generally, COPD is diagnosed later in life,8,14 when a cure of the disease or the possibility to make a significant impact is unlikely.14,15 Recent research (unpublished)16in the general population found that: 1) many factors relate to lung function throughout life; 2) these factors vary according to age; and 3) these factors interrelate. Older patients had an extremely complex interrelating network of influencing factors on lung function, which translates into an increasing complexity for treatment decision making.The current treatment strategy in COPD has been to follow the disease (e.g., the best predictor of future exacerbations is past exacerbations); Prof Agustíemphasised how it is time to start leading it.
Targeting the Sm(all) Airways in Asthma and Chronic Obstructive Pulmonary Disease
Professor Salman Siddiqui
Multiple evidence now exists supporting the role of the small airways in both asthma and COPD, yet, a deployable definition has remained elusive.17 The reason is that the small airway compartment is a difficult anatomic area to study due to its relative inaccessibility. They include the small, conducting bronchioles; the bronchiolar airways; and the acinar airways.18 Different tests measure different parts of this compartment and what we need is to move beyond individual tests to define SAD. Pathology evidence indicates small airways are strategically important in COPD. Micro-CT imaging of lung-resected tissue in patients with COPD, showed that even patients with early disease have loss of small airways (before the development of emphysema and extensive lung damage). The progression in patients across the spectrum of COPD severity was strongly associated with airway wall thickening, increasing lumen mucous exudates, and the number of airways containing acute inflammatory cells.19,20There is a growing body of evidence to show that extrafine therapies can reach the small airways. Several studies have shown that extrafine drugs can reach and are retained in the small airways and can treat them producing improved forced vital capacity, 6-minute walk distance test, and reduced exacerbations of COPD.17,21
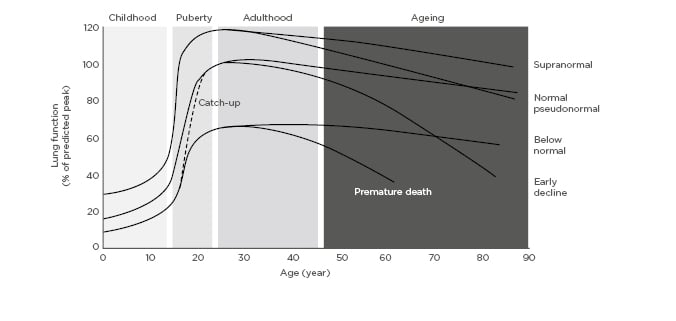
Figure 1: Lung function trajectories from birth to death.
Adapted from Agustí and Hogg.1
The same concepts apply to asthma; however, a key challenge is that no gold standard test exists to detect SAD. There are several tests that have been used to study SAD, including spirometry, impulse oscillometry (IOS), mid expiratory flows, forced vital capacity, and multiple breath nitrogen washout to measure conductive and acinar ventilation heterogeneity. IOS is particularly useful because it can be deployed across the life course, takes only a few minutes to perform, and is highly reproducible. It uses sound waves to provide a measure of airway resistance at different frequencies.22This is supported by studies, which found that IOS R5-R20 parameters correlated with the degree of morphologic abnormalities of small airways in COPD and asthma patients.23,24These findings needed confirmation from large-scale clinical trials.25ATLANTIS, a 1-year, prospective, observational, multicentre, multinational study in 800 patients with asthma across a range of severity (including smokers) and 100 healthy controls, was initiated to address this need. This is the largest study to date on SAD and the objectives were to determine the role of small airway abnormalities in the clinical manifestations of asthma, and to evaluate which clinical methods best assesses the abnormalities of small airways and large airways disease in asthma and best relates to asthma severity, control, QoL, and future risk of exacerbations; thereby, potentially establishing a method of defining SAD.26 The prevalence of SAD in asthma using all techniques was 90.7%. Gas exchange tests such as Sacin (index of diffusive ventilation heterogeneity in most peripheral pre-acinar/acinar airways) showed <20.0% prevalence of SAD (showing specificity but not sensitivity). Whilst, spirometry test (73.1% prevalence) appeared to be oversensitive, but not specific. Every patient was assigned a clinical SAD score from the model and this was used to identify SAD phenotypes using clustering. This identified two groups: patients with milder SAD and patients with severe SAD. Patients with severe SAD showed massive enrichment of abnormal oscillometry (up to 6-fold higher in some patients) when compared to those with mild SAD. When reviewing the CT scans to undertake a similar modelling, the two (physiological versus CT) did not closely correlate (potentially because of gravitational forces on lung function affecting the different way those are measured) (Figure 2).27
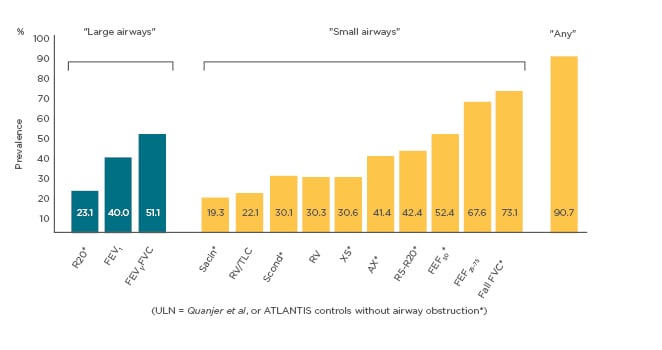
Figure 2. Prevalence of abnormal physiological variables of SAD28
Prof Siddiqui concluded that there is clear evidence to show that all of the airways, including small airways, are critically important in the pathophysiology of asthma and COPD (including early COPD) and a high prevalence of SAD in asthma and COPD. The world’s largest ATLANTIS study of SAD across asthma severities confirmed that a simple combination of oscillometry and spirometry can be used to identify SAD phenotype. Furthermore, the SAD phenotype was associated with more severe disease and adverse outcomes such as worse asthma control and a higher risk of exacerbations.
Triple Therapies: Opening Spaces for Hope in Chronic Obstructive Pulmonary Disease Patients
Professor Alberto Papi
The GOLD define COPD as “a common, preventable, and treatable disease that is characterised by persistent respiratory symptoms and airflow limitation […] (which) is caused by a mixture of small airway disease (e.g., obstructive bronchiolitis) and parenchymal destruction (emphysema), the relative contributions of which vary from person to person.”13Hogg et al.20confirmed the relationship between airflow limitation and severity of inflammatory infiltrates (innate and adaptive inflammatory immune cells) in peripheral airways.The airway inflammatory process is amplified during acute exacerbation events. GOLD defines exacerbations of COPD as an acute worsening of respiratory symptoms that requires additional medication and/or hospitalisation.13 COPD exacerbations are heterogeneous and the pathophysiology is not yet fully understood. The acute events and increased airway inflammation lead to structural changes, such as increased thickening of the airway wall, functional changes with bronchoconstriction, and increased mucus production and pathophysiology modifications (e.g., increased airflow limitation and hyperinflation) related to worsening of symptoms. The goal is to interfere with this cascade of events, at whatever level, to avoid the evolution of increased symptoms that characterise exacerbations.28,29Treatment or prevention of exacerbations are critical (and may require targeting of different pathways), due to the significant negative impact on pulmonary function, health status, QoL, risk of hospitalisation, outcomes, and healthcare costs.13In fact, the increased risk of mortality has not only been found during the acute episode but also up to 5 years after hospitalisation.30COPD exacerbations also increase the risk of cardiovascular events during and after an acute episode.31One mechanism for preventing exacerbations is the use of BD, via the effect on airway patency, but also the pathophysiological mechanisms they induce.32Treatment can be further improved with the addition of ICS as shown by a Cochrane review, which demonstrated that combination ICS therapy led to fewer exacerbations, improved QoL, lung function, and symptom scores when compared to the use of LABA monotherapy.33 GOLD has recently updated their recommendations for initial treatment in patients with GOLD D stage.13
The previous 2017 version was supported by evidence of two bronchodilators (LABA and LAMA) in combination being more effective than a single long-acting BD in preventing exacerbations.34Since new evidence has become available showing that a combination of LABA/LAMA over LAMA monotherapy for exacerbation prevention has not been consistently demonstrated, the 3 options are recommended in this group of patients as initial treatment (LAMA, ICS/LABA, preferentially in patients with BEC ≥300 cells/µL, LABA/LAMA preferentially in symptomatic patients with a COPD Assessment Test score >20.35The new GOLD concept in the follow-up treatment is a personalised approach, considering the key needs and the current treatment, to orientate decisions. The recent changes are because of new clinical evidence.13The previous 2017 GOLD recommendation came after the publication of the results of the FLAME study, which showed that at 52 weeks, LABA/LAMA was associated with a significant reduction (11%) in the annual rate of exacerbation when compared to ICS/LABA in all levels of severity.36In 2018, data from the IMPACT study demonstrated that ICS/LABA resulted in a lower rate of COPD exacerbations than LAMA/LABA (1.07 per year with ICS/LABA versus 1.21 with LABA/LAMA).37Patients enrolled in the IMPACT study had a higher frequency of exacerbations when compared to the FLAME study population and thus are the population in greater need for effective exacerbation prevention.38,39If exacerbations occur in patients treated with dual (ICS/LABA or LABA/LAMA) therapy, the next step in the GOLD exacerbation algorithm is moving to triple therapy with ICS/LABA/LAMA (with BEC ≥100 cells/µL for those on LABA/LAMA).13The evidence for this recommendation comes from the TRILOGY study, which found an adjusted annual exacerbation frequency of 0.41 for triple therapy (ICS/LABA/LAMA) and 0.53 for ICS/LABA, corresponding to a 23% reduction in exacerbations with triple therapy. The TRILOGY study, for the first time, provides 1-year evidence showing that triple therapy is superior to ICS/LABA in terms of forced expiratory volume in one second (FEV1), reduced exacerbation rate, and other symptoms-based and lung function parameters.38The subsequent TRINITY study found that exacerbation rates were 0.46 for fixed triple, 0.57 for LAMA monotherapy, and 0.45 for open triple; fixed triple was superior to LAMA (p=0.0025).39This is the first evidence to support triple therapy (either fixed or open) over LAMA monotherapy in preventing COPD exacerbations as primary outcome, which somehow raises questions of why you would escalate to dual therapy from LAMA treatment, given that the benefit of LABA/LAMA over LAMA is questionable in terms of exacerbation prevention. The TRIBUTE study further demonstrated the superior efficacy of extrafine triple therapy in reducing the rate of exacerbations as compared to LABA/LAMA (0.50 for triple and 0.59 for LABA/LAMA). This translates into a 15.2% reduction of moderate/severe COPD exacerbation risk for triple versus dual therapy. Triple therapy was also superior to LABA/LAMA in pre-dose FEV1 and improvement in QoL.40In addition, the IMPACT study showed that the annual rate of severe exacerbations resulting in hospitalisation in the triple-therapy group was 0.13 compared to 0.19 in the LAMA/LABA group (p<0.001).37Adverse events were similar across treatment groups.40Pneumonia was reported in a small number of patients across all three clinical trials (TRINITY, TRILOGY, and TRIBUTE), with similar incidences. This data show that the balance between the benefits of the reduction in COPD exacerbation versus the risk of development of pneumonia is 7–10-fold in favour of the benefits.38-41 This is aligned with the European Medicines Agency (EMA) Pharmacovigilance Risk Assessment Committee (PRAC) review that the benefits of ICS continue to outweigh their risks.42Triple therapy, therefore, provides new treatment options and potentially new hopes for patients with moderate-to-severe COPD exacerbations. Triple therapy may provide the opportunity to lead this disease. Prof Papi questioned the need to wait for 2 exacerbations to escalate treatment when there is evidence showing that exacerbation risk is more than doubled in patients experiencing only 1 exacerbation in the past 12 months,43considering that in the last years consistent evidence has become available showing the efficacy of ICS/LABA over LABA and of triple over dual combinations in preventing exacerbations episodes in COPD patients that have exacerbated once in the previous year (Figure 3).37,44,45A reduction in mortality rates using triple therapy has also been reported in the IMPACT study.37,46Moreover, pooled data from TRILOGY, TRINITY, and TRIBUTE studies on fatal events showed a clear trend towards the reduction of mortality risk with triple therapy.46
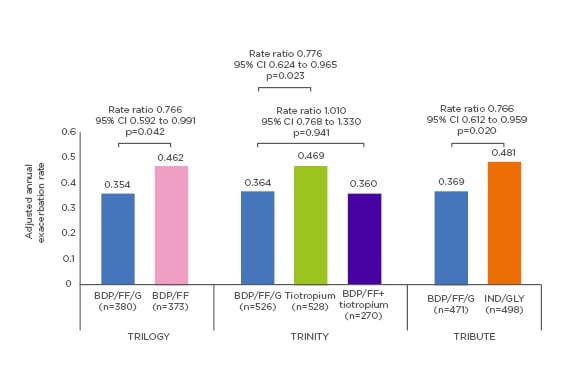
Figure 3: Adjusted annual rate of moderate-to-severe chronic obstructive pulmonary disease exacerbations (intention-to-treat population).
Triple therapy shows superior efficacy in reducing moderate to severe chronic obstructive pulmonary disease exacerbations.
BDP: beclometasone dipropionate; CI: confidence interval; FF: formoterol fumarate; G: glycopyrronium bromide; GLY: Glycopyrronium; IND: Indacaterol.
Adapted from Singh et al.45
Inhaled Corticosteroid for many or Inhaled Corticosteroid for Some.Inspiration comes from Adaptation
Professor Bartolome R Celli and Professor Dave Singh
Prof Celli opened the debate by outlining that guidelines do not recommend the use of ICS alone in COPD.13The reason for this is that well-conducted studies have shown that ICS, when used as a monotherapy, is not very effective in COPD. The TORCH study in which ≥6,000 patients over 3 years compared combination therapy (ICS/LABA) versus placebo, LABA, and ICS monotherapy. The primary outcome was death from any cause, and the all-cause mortality rates for salmeterol or fluticasone propionate alone did not differ significantly from placebo. However, there was a statistically significant higher mortality rate in the fluticasone arm alone compared with the LABA/ICS arm.47 Several studies have shown that when ICS is added to BD (either LABA or LABA+LAMA), the efficacy in different COPD outcomes is proven.38-41The goals for treatment outlined by the GOLD initiative are to reduce symptoms (improve symptoms, exercise tolerance, and health status) and risks (disease progression, exacerbations, and mortality).13This should be implemented in combination with the Hippocratic oath to “abstain from doing harm”. The TORCH study showed that the ICS-combination regimen significantly improved QoL and spirometric values.47,48Furthermore, the SUMMIT trial including >16,500 patients showed ICS-containing regimens (ICS/LABA) had significantly lower adjusted rates of FEV1 decline versus placebo (p<0.03).49 In TORCH, the ICS-combination regimen reduced the annual rate of exacerbations from 1.13 to 0.85.47GOLD advocates that you need to have an exacerbation to be treated with ICS therapy.13However, SUMMIT demonstrated that patients with moderate, chronic airflow obstruction, experienced a reduction in moderate and severe exacerbations with ICS/LABA combination compared with placebo, irrespective of a history of exacerbations or baseline FEV1. In fact, >60% of the patients had no exacerbations in the year prior to study inclusion.49,50In addition, the TRIBUTE study showed that treatment with the extrafine single inhaler triple combination significantly reduced the rate of moderate-to-severe exacerbations compared with LABA/LAMA in severe and very severe COPD patients with a history of at least one or more moderate and severe exacerbation the year prior to enrolment.40 Furthermore, as shown from Singh et al.,51treatment with extrafine triple delayed disease deterioration compared to LABA/LAMA in the TRIBUTE study. An understated argument for the use of ICS is its potential impact on mortality. Indeed, the reduction in the risk of death was found to be 17.5% in TORCH (p=0.052),47 12% in SUMMIT (p=0.137),5242% in IMPACT (p=0.01),37and the pooled TRINITY, TRILOGY, TRIBUTE data showed a 28% reduction in the risk of death (p=0.066) with ICS/LABA/LAMA.46There is no question that the use of ICS increases the risk of pneumonia, however, this depends on the type of ICS used, the dose, the severity of airflow limitation, and the age and nutritional status of the patients. Interestingly, the incidence of severe pneumonia was similar amongst therapies in the TRIBUTE study.40 Moreover, Festic et al.53found that despite the association of ICS with increased risk of pneumonia, their use has not been associated with increased risk of pneumonia-related or overall mortality, suggesting a possible double effect of ICS (i.e., an adverse effect plus an unexplained mitigating effect). Bafadhel et al.54postulated that BEC (≥100 cells/µL) might identify COPD patients taking ICS who will experience fewer exacerbations, with a continuous relationship between levels of eosinophils in the blood and ICS response in clinical trials.54This is supported by other studies.55-57This threshold of 100 cells/µL could potentially identify which patients are not suitable for ICS therapy and guide personalised medicine. These studies highlight that ICS-combination therapy can improve health status,47prevent disease progression,48-49prevent and treat exacerbations,47,50and reduce mortality.37,46,47
Prof Singh then countered by stating that using only group mean data from clinical trials to determine treatment approach is not precision medicine, and that there is more complexity to the art of medicine for individual patients. There is a huge variation in ICS response in asthma patients as shown by Martin et al.58in the distribution of the percent change in FEV1. Clinicians know that the benefit of ICS treatment also varies between COPD patients and that the challenge is to identify individuals in whom the benefits outweigh the risks. There is an interaction between the exacerbation risk and BEC for the individual patient. BEC clearly predict the responders versus nonresponders for ICS. Siddiqui et al.59showed that with lowering BEC, the adjusted reduction rate in COPD exacerbation decreased. Bafadhel et al.54showed this using binomial regression, that at around BEC=300 cells/µL, the reduction in ICS effect is approximately 50% and below BEC=100 cells/µL, appears to be no ICS effect.54However, this is a continuous relationship without definitive threshold values for response or nonresponse; instead, BEC but probabilities of response, i.e., with increasing BEC, the probability of an ICS response increases. GOLD therefore recommends using this biomarker as part of clinical decision making to provide an estimate of the probability of response to ICS.13Modelling BEC and response to triple and double combinations provide similar results regarding ICS treatment.60Kolsum et al.61conducted a bronchoscopy study to evaluate differences in airway biology associated with BEC and found that higher BEC had parallel increases in lung eosinophil counts, and other various inflammatory proteins. Furthermore, another study showed that patients with sputum eosinophil counts had less colonising bacteria.62Overall these findings show differences in inflammation and microbiome that could explain different responses to ICS. Furthermore, patients with more frequent exacerbations, have the highest potential for ICS benefits.63Those patients with less frequent exacerbations, but higher BEC will also benefit from ICS. So, in the age of personalised medicine, the point at which you would use an ICS inhaler depends on the complexity of clinical and biological information. This complexity of information includes the risk of adverse events.64-66The risk of pneumonia is personalised, with well-known risk factors, including increasing age, lower BMI, and history of pneumonia.67 The regulators agree that the benefits of ICS outweigh the risks.42Prof Singh stressed that ICS effects are related to dose, there is no reason to use high dose ICS in COPD. Real-world data comparing extrafine formulation and fine-particle ICS found that extrafine formulation ICS/LABA (which deliver a lower dose due to efficient drug delivery) had significantly lower pneumonia risk.68Therefore, GOLD recommends a personalised treatment algorithm based on exacerbation risk and BEC, to treat some patients with ICS.13In conclusion, precision medicine is using all of the clinical and biological information available to make the right decision for the
right patient.
Conclusion
Clinicians are facing the new challenges in airway diseases, particularly the changing paradigm toward precision medicine, addressing the complexity of this disease specifically in the ageing population and the critical need to treat COPD early to prevent future events and delay disease deterioration. Fixed triple therapy may help address these challenges with proven reductions in exacerbations, improvement in lung function, symptoms, and QoL, and reduction in the risk of mortality. These benefits far outweigh the risks of adverse events, including pneumonia. Extrafine fixed triple therapy moreover is able to reach both large and small airways, that are critically important in the pathophysiology of asthma and COPD.








