Chairpeople: Felix Herth,1 Daiana Stolz2
Speakers: Felix Herth, Maria Sucena,3 Ilaria Ferrarotti,4 Dave Singh5
1. Department of Pneumology and Critical Care Medicine, Thoraxklinik-Heidelberg; Translational Lung Research Center (TRCH), University of Heidelberg, Germany
2. Clinic for Respiratory Medicine and Pulmonary Cell Research, University Hospital Basel, Switzerland
3. Pulmonology Department, Centro Hospitalar Universitário do Porto, Portugal
4. Centre for Diagnosis of Inherited Alpha 1 Antitrypsin Deficiency, Università di Pavia, Lombardy, Italy
5. Clinic of Pneumology, University Hospital Freiburg, Germany
Disclosure: Herth is affiliated with, or has received grants or research support from the German Federal Ministry of Education and Research (BMBF), BMG Pharma, Broncus-Uptake Medical, Deutsche Forschungsgemeinschaft (DFG), the European Union (EU), Klaus Tschirra Stiftung, Olympus Medical Systems, Pulmonx, and Roche Diagnostics; honoraria or consulting fees from AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Chiesi Farmaceutici S.p.A., Erbe China, Novartis, MedUpdates, Pulmonx, Roche Diagnostics, Uptake Medical, Boston Scientific, Broncus-Uptake Medical, Dinova Pharmaceutical Inc, Erbe Medical, Free Flow Medical, Johnson & Johnson, Karger Publishers, LAK Medical, Nanovation, and Olympus Medical. Stolz has declared no conflicts of interest. Sucena has received speaker or consulting fees from CSL Behring, Grifols, Boehringer Ingelheim, and Novartis; and personal fees from Bial, Boehringer Ingelheim, CSL Behring, DarSaúde, Gasoxmed, Grifols, Linde, Menarini, Novartis, and VitalAire. Ferrarotti has received grants or research support from CSL Behring and has participated in a company-sponsored bureau for Grifols. Singh has received personal fees from AstraZeneca, Aerogen, Boehringer Ingelheim, Chiesi Farmaceutici S.p.A., Cipla, CSL Behring, EpiEndo, Genentech, GlaxoSmithKline, Glenmark Pharmaceutical, Gossamer Bio, Kinaset Therapeutics, Menarini, Novartis, PULMATRiX, Sanofi, Teva Pharmaceutical, Theravance Biopharma, and Verona Pharma.
Acknowledgements: Writing assistance was provided by Nicola Humphry, Nottingham, UK.
Support: The symposium and publication of this article were funded by CSL Behring. The views and opinions expressed are exclusively those of the speakers
Citation: EMJ Respir. 2021;9[1]:31-39.
Meeting Summary
Maria Sucena opened the symposium by emphasising the importance of establishing the priority for research into alpha 1 antitrypsin deficiency (AATD) and promoting collaboration at a national and international level. She described several projects of the European Alpha-1 Research Collaboration (EARCO), which aim to address these needs, including the creation of a pan-European AATD registry and the identification of research priorities in AATD. Ilaria Ferrarotti followed by describing three key learnings from recent reports on current diagnostic methods. These include an unexpectedly high rate of rare mutations in the SERPINA1 gene, which requires optimised techniques in the diagnosis of AATD, variable accuracies of published diagnostic algorithms, and findings that suggest that a low alpha 1 fraction in routine serum protein electrophoresis (SPE) should lead to a suspicion of AATD. Felix Herth then discussed the importance of maintaining AAT therapy in patients with AATD. He explained that self-administration promotes independence in suitable patients, and that it has the potential to overcome some of the challenges for AAT therapy during the COVID-19 pandemic. Lastly, Dave Singh summarised the literature on COVID-19 and pulmonary disease, explaining that, overall, data suggests that COVID-19 prevalence and outcomes are worse in patients with pulmonary disease, particularly chronic obstructive pulmonary disease (COPD). He clarified that despite confounding factors in studies that have analysed the risks associated with inhaled corticosteroid, there does not appear to be clear evidence that these medicines should be withheld.
Planning for Success: Research Priorities in Alpha 1 Antitrypsin Deficiency
Maria Sucena
Sucena emphasised that although great improvements have been made in the understanding of AATD in recent years, many questions remain unanswered. She explained that for a successful future, it is imperative to establish the priorities for research into this disease. To facilitate high quality research in a rare disease such as AATD, collaboration at a national and international level is needed.
The European Respiratory Society (ERS) established the EARCO to bring together a network of researchers, patients, and clinical experts in AATD to guide clinical and research priorities in Europe.1 The EARCO aims to support and encourage early career researchers, and to increase the number and quality of clinical trials performed in AATD.1
Sucena explained that the first objective of the EARCO is a pan-European AATD registry, with a quality control system ensuring high quality and completeness of data entered into the database: the EARCO registry.1 The enrolment of patients with AATD began early in 2020 and, despite a pause in recruitment due to the COVID-19 pandemic, there are now over 40 sites currently recruiting patients across Europe, with an aim of including more than 3,000 patients in the registry in the first 3 years.2 The key criteria for inclusion in the EARCO registry are AATD with an AAT serum level of <11 µM (50 mg/dL) and/or a proteinase inhibitor genotype ZZ, SZ, or compound heterozygotes or homozygotes of other rare deficient variants. Data collected include sociodemographics, respiratory physiology, radiology, blood tests, symptoms, exacerbations, comorbidities, and treatment.2
Sucena described the results from another EARCO project: the identification of research priorities in AATD, from the perspective of patients, carers, and healthcare providers (HCPs). For this project, two surveys were developed: one for HCPs and another for patients and caregivers. The HCPs’ survey was sent to AATD experts throughout Europe, with 94 respondents across 24 countries. The survey for patients and caregivers was translated into nine languages; 438 questionnaires were completed by individuals across 26 countries.3
The top five most important research questions rated by HCPs were: the causes of fast progression and poor outcome in patients with AATD; improvement of early and accurate diagnosis of AATD; time for initiation of AAT therapy; effectiveness of self-management interventions; and optimal dose regimen of AAT therapy.3
The patients and carers considered the most challenging aspects of AATD to be decreased exercise tolerance and shortness of breath, followed by not feeling fit or having the strength to do daily activities, tiredness, and fatigue. They considered the most challenging aspects for treatment to be issues with access to AAT therapy, the professional implications of the diagnosis of AATD, and the access to classes or fitness centres after rehabilitation. In terms of research priorities for AATD, patients and carers considered the top priority to be improving the knowledge of AATD, particularly among general practitioners, with 99% of patients rating this as an important or very important area. Other important priorities included: access to AATD specialised centres; access to reliable, easy to understand information about living with AATD; being able to recognise an exacerbation; and targeted screening programmes for COPD and asthma patients.3
Figure 1 presents the collective research and disease management priorities identified by HCPs, patients, and carers.3
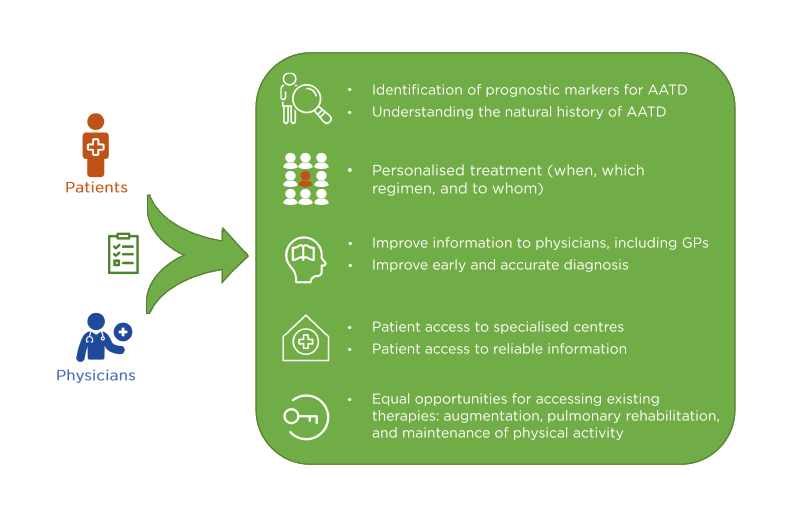
Figure 1: Top research and management priorities identified by healthcare professionals and patients in alpha 1 antitrypsin deficiency.
AATD: alpha 1 antitrypsin deficiency; GP: general practitioner. Adapted from Barrecheguren et al.3
Sucena explained that an EARCO working group is now planning a 5-year strategy to provide evidence to address these issues. From just a few AATD researchers, HCPs, patients, and industry partners, the EARCO network has grown to include representatives from almost all European countries.
What Are the Future Plans of the Research Co-operation?
The EARCO plans to investigate the impact of COVID-19 and bronchiectasis on patients with AATD, and to gather expert opinions on the initiation of AAT therapy.
Are All Questions in the Registry Mandatory?
The EARCO registry will include patients from many different countries; therefore, only the sociodemographic data and basic information about lung and liver disease will be mandatory.
Optimising Laboratory Diagnosis of Alpha 1 Antitrypsin Deficiency
Ilaria Ferrarotti
Ferrarotti began by explaining that since AATD is a rare condition, it is logical to ask how diagnosis can be optimised.
Firstly, Ferrarotti emphasised that the SERPINA1 gene that encodes AAT is highly polymorphic. Aside from the most common S and Z mutations, there are many other pathological alleles.4 Recent studies of National Registries in Ireland and Italy and have reported rare variant rates of 5.8% and 21.3%, respectively,5,6 and in Ferrarotti’s own centre in Pavia, 43.0–44.0% of AATD cases over the last 3 years were caused by rare genotypes. Together, these data suggest that actual prevalence of AATD could be much higher than suggested by the estimates based on the ZZ-genotype.
The second issue pertaining to laboratory diagnosis of AATD is that diagnostic testing methods can vary and many algorithms utilising these methods have been proposed for the diagnosis of AATD.4
To compare the efficacy in detecting AATD, Ferrarotti and her team carried out a systematic literature review to select six well-described diagnostic algorithms.4 Each algorithm was then retrospectively applied to >5,000 samples from fully characterised patients diagnosed with AATD in Pavia. The study found that the frequency of false negatives varied between 1.9–12.9% and the rate of true positives ranged from 24.2–35.1%.4 Ferrarotti emphasised that the choice of diagnostic algorithm is critical for the accurate diagnosis of AATD, and that the potential for false negatives should highlight to clinicians the importance of repeating tests, or adjusting the diagnostic algorithm, if a negative result occurs in a patient with clinically suspected AATD.
Lastly, Ferrarotti discussed the findings of a study into the potential of reflex testing for AATD based on the alpha 1 protein fraction of SP.7 Investigators screened samples from >20,000 subjects and identified 82 cases with reduced alpha 1 globulin levels; these were then genotyped for SERPINA1 mutation. The detection rate of the composite algorithm was 51%, whereas standard screening procedures, based on clinical profiling and AAT levels <1 g/L resulted in a detection rate of just 19%.7 A similar approach was used in a centre in Ireland, whereby any SPE with a low alpha 1 fraction was reflexively tested for serum AAT levels over 32 months. Over >65,000 SPE tests were analysed, of which 360 had low alpha 1 fractions and underwent testing for AAT. This approach resulted in a high diagnostic rate of 39%, compared with a diagnostic rate of 58% with standard clinically targeted testing.8
Ferrarotti concluded by reiterating that AATD can be caused by a rare mutation in the SERPINA1 gene, and diagnostic approaches should take this into account; that the choice of diagnostic algorithm has a significant impact on the correct diagnosis of AATD; and that a low alpha 1 fraction in SPE should lead to a suspicion of AATD, which should be confirmed by further analysis.
How Does EARCO Help Us to Improve the Care of Patients with Alpha 1 Antitrypsin Deficiency?
EARCO activities have organised a network of seven leading laboratories across Europe. This enables the sharing of material, quality control checking, and harmonisation of the diagnostic activity of each laboratory.
Is the Measurement of the Alpha 1 Antitrypsin Level Sufficient to Diagnose Alpha 1 Antitrypsin Deficiency?
This is just the first step towards diagnosis. In most cases, it is insufficient to diagnose AATD.
Paradigm Shifts in Alpha 1 Antitrypsin Deficiency: The Impact of COVID-19 on Patient Management
Felix Herth
The benefits of AAT augmentation therapy in patients with AATD have been demonstrated in multiple clinical trials,9 and abrupt cessation of AAT therapy has been shown to result in a rapid increase in the rate of exacerbations over a matter of weeks.10 Herth stressed that once AAT therapy has started, it must be continued to control exacerbation rates and, therefore, reduce the progression of the disease.
Research suggests that patients with AATD may have an increased risk of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and a higher risk of worse outcomes compared with the general population, due to the underlying pathophysiology of AATD and AATD-related comorbidities.11 Herth explained that the risk of infection has caused patients to feel uncertain about continuing their AAT therapy because it requires contact with the healthcare system. Together with the limited access to healthcare systems at the start of the pandemic, this resulted in the abrupt cessation of treatment in some patients.
To address these challenges, clinicians have implemented additional safety measures in hospitals and clinics to mitigate the risk of SARS-CoV-2 transmission. These changes have had the additional benefit of limiting the number of exacerbations in patients with AATD. In addition, increasing numbers of patients have been referred for self-administration therapy, with virtual training sessions.
In the USA, patients who self-administer their AAT treatment consider the main benefit to be greater independence.12 Herth posited that self-administration could potentially address some of the challenges of AAT therapy during the COVID-19 pandemic by minimising contact with HCPs and the general public. Herth suggested that intravenous infusion training sessions could be conducted virtually, and online resources and emergency contacts could be made available to patients.13 Although a survey of physicians in Europe suggested that the ‘ideal’ patient for self-administration of AAT therapy would be younger, more stable, and with less comorbidities than other patients,14 Herth feels that the older patient, with more comorbidities, may also be suitable with additional training and support. He provided an example of a 52-year-old patient from his own practice who had been undergoing AAT therapy since 2016. She discontinued therapy in March 2020 due to the COVID-19 pandemic and experienced an exacerbation 6 weeks later. After a discussion with the patient, she was given three training sessions, and has since been successfully self-administering AAT therapy at home (Figure 2). Herth emphasised that self-administration training should continue during the pandemic, provided that patients are adequately trained, training is delivered virtually, and patients are trained between exacerbations.
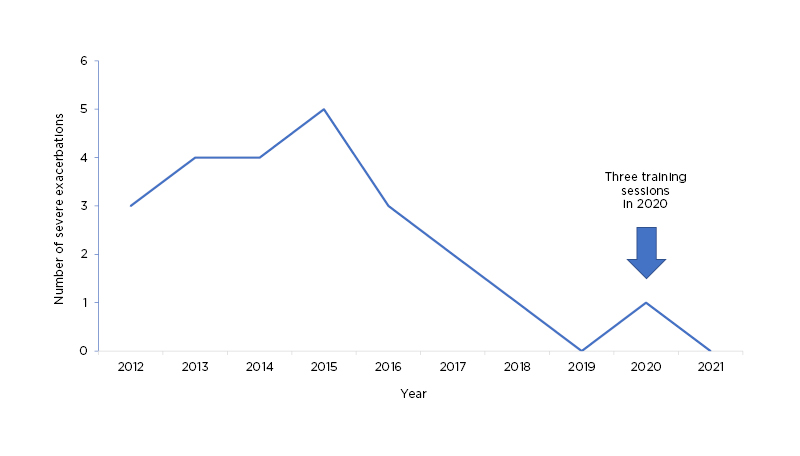
Figure 2: Exacerbations experienced by a patient with alpha 1 antitrypsin deficiency who initiated self-administration of alpha 1 antitrypsin therapy.
In summary, Herth reiterated that it is important to ensure the continuation of AAT therapy in patients with AATD, that self-administration is an alternative means of treatment delivery that promotes independence in appropriate patients, and self-administration is a viable option for most patients during the COVID-19 pandemic.
What Do You Think Was the Most Significant Challenge to the Treatment and Care of Patients with Alpha 1 Antitrypsin Deficiency During the COVID-19 Pandemic?
In many countries, patients were concerned that they would become infected with SARS-CoV-2 if they visited their doctor. Herth explained that his clinic persuaded some of the more cautious patients to visit the clinic to learn about self-administration. Prior to COVID-19, about 10–15% of Herth’s patients were using self-administration of AAT, but these figures have grown to 25–30%.
How Can You Be Sure that Self-Administration Is Working Safely and Correctly?
There is sometimes a concern that self-administration puts clinicians at risk of litigation if something goes wrong. Herth explained that, provided a suitable training regime is followed, this is not an issue. In Herth’s experience, those patients who go through the training are usually very compliant in administering their treatment correctly at home.
Strategies to Prevent and Manage Severe COVID-19
Dave Singh
Singh introduced his topic by describing several large cohort studies conducted during the first wave of the COVID19 pandemic. Hippisley-Cox et al. investigated the risk of severe COVID-19 disease in patients taking angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers,15 including individuals with concomitant asthma (13.0%) and COPD (2.4%). A small proportionate number of patients with asthma (14.0%) were represented in those admitted to an intensive care unit with COVID-19, suggesting that asthma did not represent a risk factor for severe COVID-19 disease. However, patients with COPD made up a disproportionate number of those admitted to an intensive care unit (3.6%), which provided one of the first indications that patients with COPD were at increased risk of severe COVID-19. In the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) World Health Organization (WHO) Clinical Characterisation Protocol (CCP)-UK study, both asthma and chronic pulmonary disease were among the most common comorbidities observed in patients admitted to hospital with COVID-19 in the UK.16 In a review of the impact of COPD on the clinical outcomes of COVID-19, most studies were found to report an increased risk for severe COVID-19 in patients with COPD.17
Singh explained that patients with COPD exhibit upregulated levels of proteins and receptors involved in the processing and uptake of the SARS-CoV-2 virus in epithelial cells compared to controls, notably ACE2, with less evidence for other proteins such as furin and transmembrane protease serine 2 precursor (TMPRSS2).17 Singh posited that patients with COPD may, therefore, be predisposed to a high viral load.
There is some debate among experts about whether corticosteroids used to treat COPD and asthma increase or decrease this susceptibility. Inhaled corticosteroids can downregulate interferon-β, thereby enhancing viral replication, and also reduce anti-bacterial defence.18 On the other hand, inhaled corticosteroids appear to downregulate ACE2 and TMPRSS2 in patients with asthma,19 which could, theoretically, reduce the ability of SARS-CoV-2 to enter epithelial cells.
Singh explained that, overall, the literature indicates that COVID-19 prevalence and outcomes are worse for pulmonary disease, particularly in COPD.20-23 It is difficult to determine the risks associated with inhaled corticosteroid use due to confounding factors, but there does not appear to be clear evidence that these medicines are harmful.
In the second part of his presentation, Singh discussed interventions to manage and prevent severe COVID-19, beginning with the large randomised controlled trials that support the current management of COVID-19.
Data from the RECOVERY trial, conducted in the UK, showed that dexamethasone treatment reduced COVID-19-related 28-day mortality compared with usual care.24 However, this benefit only applied to patients who were given oxygen or invasive mechanical ventilation on admission to hospital. Another analysis of the same trial showed that tocilizumab reduced 28-day mortality in a sub-group of hospitalised patients with COVID-19 who were hypoxic on room air (oxygen saturation: <92%) and had high C-reactive protein levels (≥75 mg/L), compared with usual care.25 Lastly, a clinical trial of the oral JAK inhibitor baricitinib plus anti-viral agent remdesivir in patients hospitalised with COVID-19, analysed the patients’ time to recovery. Overall, combination treatment was superior to remdesivir alone, particularly in patients who required non-invasive ventilation or high-flow oxygen.26 Other studies have suggested that remdesivir monotherapy is also associated with an improved time to recovery,27 but not a reduction in overall mortality in hospitalised patients.28
Singh explained that these data suggest that there is a disconnection between the clinical trial endpoints of recovery and mortality, which may suggest that anti-viral agents are more effective in the earlier stages of COVID-19 compared with the more severe, later stages. This implies that COVID-19 therapy is moving towards a more personalised, precision-medicine approach, where treatments are targeted to patients according to their clinical characteristics (including disease severity) and biomarkers (indicating the level of inflammation). For patients who are not hypoxic, Singh clarified that there are not yet treatments that can halt progression of the disease. However, in other patients there is good evidence that dexamethasone can be used, with the addition of anti-IL-6 in those with more inflammation, and these two therapies form the basis of current treatment for COVID-19. JAK inhibitors also show promise as treatment for patients with more severe COVID-19.
Other therapeutic modalities include the tyrosine kinase inhibitor imatinib, which reduces vascular leak, and has the potential to attenuate the pulmonary oedema, which occurs during severe COVID-19. While the primary endpoint, discontinuation of ventilation and supplemental oxygen, did not reveal a beneficial effect, the mortality benefits were clear (hazard ratio: 0.52).29 In a small, Phase II trial, inhaled interferon-β1a, an antiviral therapy, improved recovery from SARS-CoV-2 infection,30 and this molecule has progressed to larger, Phase III studies.31 Lastly, the STOIC Phase II trial of community-administered budesonide at an early stage of COVID-19 suggests that this inhaled corticosteroid improves the time to clinical recovery.32
Singh explained that, together, the beneficial effects of dexamethasone in patients hospitalised with COVID-19, and the faster recovery from early COVID-19 induced by budesonide in the community, suggest a protective effect of corticosteroids in patients with asthma and COPD.
Finally, Singh summarised that comorbidities in patients with AATD, including hypertension, chronic kidney disease, diabetes, and COPD, are risk factors for COVID-19. Interestingly, alpha 1 proteinase inhibitors might inhibit SARS-CoV-2 uptake into cells and, along with anticoagulant and anti-inflammatory activity, this suggests that these agents could be protective against severe COVID-19, and there are several ongoing clinical trials to test this hypothesis11 (Figure 3).
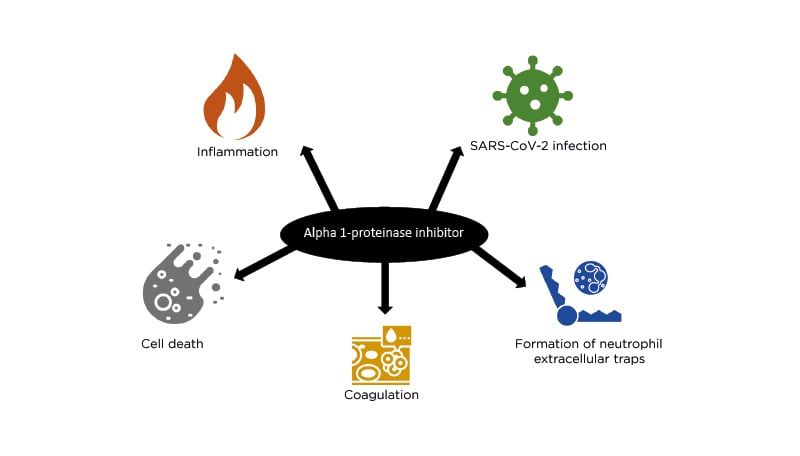
Figure 3: Alpha 1 proteinase inhibitor and COVID-19.
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2. Adapted from Yang et al.11
What Are Your Thoughts About the Relationship Between Pulmonary Fibrosis and COVID-19?
The key is to prevent the development of severe damage from COVID-19 in the first place, and this is something which is beginning to be achieved through vaccination and good therapeutics. In many individuals, COVID-19-induced lung damage does seem to resolve over time, and in those patients with significant lung tissue damage and fibrosis, the development of therapeutics for use during ‘long COVID’ will be important.
There Are Some Contradictory Data on the Use of Anti-IL-6 Treatment. Only the Studies that Combined these Drugs with Dexamethasone Showed Positive Results. How Do You Interpret these Findings?
In both the RECOVERY study25 and the REMAP-CAP trial,33 most patients received concomitant oral corticosteroids as per standard-of-care treatment; other studies demonstrated a benefit of anti-IL-6 in patients with COVID-19. The treatment of the SARS-CoV-2 infection has evolved to include different combinations of therapies, and any new COVID-19 treatment should be expected to be tested with, and administered on top of standard-of-care therapies, in a personalised manner.
Closing remarks
Daiana Stolz
Stolz provided a brief overview of the current understanding regarding long COVID, explaining that this represents persistent lung dysfunction after SARS-CoV-2 infection in some patients, including a deterioration in lung function diffusion capacity.34 In the early stages of recovery, conditioning probably plays a major role in reduced exercise capacity, but HCPs are yet to explain the longer-term effects after SARS-CoV-2 infection. Therefore, it is important that data continue to be collected, including exercise capacity, in these patients.
Stolz concluded the symposium by clarifying that it is important to continue providing appropriate care for patients with respiratory disease during the COVID-19 pandemic. This may include the use of telemedicine, but not to the exclusion of monitoring and treating these patients. There are several initiatives in Europe that are focusing on this patient population to help HCPs to provide the best possible care. Stoltz emphasised that she was very proud to be part of a pulmonary community that has shown that it can achieve fast-paced scientific progress despite the pandemic.








