Meeting Summary
Asthma is one of the most common chronic diseases, with ≤25% of patients experiencing uncontrolled disease.1Patients with uncontrolled, moderate-to-severe asthma are at increased risk of recurrent exacerbations, accelerated decline in lung function, fixed airway obstruction, and have increased utilisation of health care resources.2,3Furthermore, reduced lung function, as assessed by measures such as forced expiratory volume in 1 second (FEV1), is a strong independent predictor of exacerbations, progressive decline in lung function, and all-cause pulmonary and cardiovascular mortality in patients with asthma.2 Achieving asthma control in these patients is therefore critical. The recognition of distinct inflammatory phenotypes within this population has been instrumental in addressing this need. In these patients, there is robust evidence of the pathogenic role of Th2 cytokines, such as IL-4 and IL-13, in the eosinophilic and allergic inflammatory processes.4This in turn has driven the development of targeted biological therapies, particularly selective targeted monoclonal antibodies such as dupilumab which inhibit the biological effects of both IL-4 and IL-13.5
This article reviews four posters displayed at the European Respiratory Society (ERS) International Congress 2019 that presented results demonstrating the efficacy and safety of dupilumab, an anti-IL-4 receptor human monoclonal antibody, compared to placebo for the treatment of uncontrolled, moderate-to-severe asthma, as measured by a range of outcomes.
Background
Atypical production of several Th2 cytokines, including IL-4, IL-5, and IL-13, play a central pathogenic role in multiple atopic conditions.6-9Specifically, IL-4 and IL-13 are associated with the pathogenesis of certain types of asthma, including allergic and nonallergic forms.5,6,9-12 IL-4 and IL-13 were historically thought to mediate identical signalling pathways because they share receptor complexes; however, IL-4 and IL-13 elicit distinct allergic hallmarks.IL‑4 is the central mediator of Th2 cell differentiation, isotype class switching (especially to IgE), B cell growth, and eosinophil (EoS) recruitment.12-14IL‑13 has roles in goblet cell hyperplasia induction and smooth muscle contractility.9,15Therefore, IL-4 and IL-13 activate multiple cell types and induce various mediators involved in inflammation, contributing to airflow limitation and increasing the risk of severe exacerbations.11-15Little is currently known regarding the roles of IL-4/IL-13 in IgE and non-IgE-mediated inflammatory pathways, or the effect of inhibiting IL-4/IL-13 in these pathways in asthma.
Dupilumab, a fully human anti-IL-4Rαmonoclonal antibody, inhibits signalling of both IL-4 and IL-13 by specifically binding to the IL-4Rαsubunit shared by both receptor complexes.5,10-12This effect is associated with the marked suppression of biomarkers of Type 2 inflammation including total serum IgE, thymus and activation regulated chemokine, eotaxin-3, and fractional exhaled nitric oxide (FeNO).13
Liberty Asthma QUEST Trial
The Liberty Asthma QUEST16was a Phase III, randomised, placebo-controlled, parallel-group trial in 1,902 patients with persistent asthma, receiving continuous inhaled corticosteroids (ICS), plus up to two additional controller medications.17Patients with uncontrolled, moderate-to-severe asthma (based on the Global Initiative for Asthma [GINA] 2015 guidelines),18with a history of one or more exacerbations in the previous year and without a minimum requirement for baseline blood EoS count or any other Type 2 biomarkers (FeNO or serum total lgE),19were randomised in a 2:2:1:1 ratio to receive 52 weeks of add-on therapy with subcutaneously administered dupilumab 200 mg or 300 mg every 2 weeks, or matched placebo.5
The co-primary efficacy endpoints included an annualised rate of severe exacerbation events during the 52-week treatmentperiod and absolute change from baseline in pre-bronchodilator (BD) FEV1at Week 12. A secondary endpoint was the percentage change from baseline to Week 12 in pre-BD FEV1.19
This study showed that add-on dupilumab significantly reduced severe asthma exacerbations; improved lung function, asthma control, and quality-of-life measures; and was generally well-tolerated.13 Moreover, treatment effects were greater in patients with elevated Type 2 biomarkers at baseline (blood EoS and FeNO).17,19
Dupilumab Effect on Lung Function in Patients with Uncontrolled, Moderate-to-Severe Asthma with an Allergic Phenotype
Professor Mario Castro
This post hoc subset analysis of the Liberty Asthma QUEST trial assessed the effect of dupilumab on lung function parameters in patients with uncontrolled, moderate-to-severe asthma with and without evidence of allergic asthma. In this study, allergic asthma was defined as total serum IgE ≥30.00 IU/mL and ≥1.00 perennial aeroallergen-specific IgE ≥0.35 kU/L. The study assessments included the change from baseline in pre-BD FEV1(L), post-BD FEV1(L), pre-BD forced expiratory flow at 25–75% of pulmonary volume (FEF25–75%, L/s), and pre-BD FEV1/forced vital capacity (FVC) ratio (%) during the 52-week treatment period in patients receiving dupilumab 200 mg every 2 weeks, 300 mg every 2 weeks, or matched placebos stratified by evidence of allergic asthma.20
Of the patients, 57% had allergic asthma (n=1,083) with a mean age of 44.40 years, 58.40% were female, and the mean number of severe exacerbations was 1.96. In the nonallergic asthma group, the mean age was 52.70 years, 70.40% were female, and the mean number of severe exacerbations was 2.32.20
This post hoc analysis showed that dupilumab improved pre and post-BD FEV1, pre-BD FEF25-75% (L/s), and FEV1/FVC ratio (%) at Weeks 12 and 52 in patients with uncontrolled, moderate-to-severe asthma with and without evidence of allergic asthma. Dupilumab 200 mg and 300 mg every 2 weeks versus placebo also improved lung function parameters at Week 12 (change from baseline least squares [LS] mean difference pre-BD FEV1:0.13/0.16 L; post-BD FEV1:0.13/0.11 L; FEF25–75%: 0.14/0.22 L/s; FVC: 0.15/0.11 L; FEV1/FVC ratio: 0.56/2.78%; all p<0.05 except dupilumab 200mg, FEV1/FVC ratio [p=0.35]). Sustained or better improvements were observed at Week 52 (all p<0.05).20
The incidence of treatment-emergent adverse events (TEAE) was similar across treatment groups and the most common TEAE reported were viral upper respiratory tract infections (18.2% versus 19.6%), injection-site erythema (13.8% versus 5.5%), upper respiratory tract infection (11.6% versus 13.6%), and bronchitis (11.4% versus 14.0%) in dupilumab versus placebo, respectively.20
Prof Castro concluded that in addition to reducing severe asthma exacerbations and biomarkers of Type 2 inflammation, including total serum IgE,21dupilumab therapy demonstrated rapid and sustained improvement in lung function in uncontrolled, moderate-to-severe asthma patients, with or without evidence of allergic asthma, during the 52-week treatment period. Dupilumab improved both large (pre and post-BD FEV1) and small (pre-BD FEF25-75%)airway function, as well as airway obstruction (pre-BD FEV1 /FVC). The magnitude of improvement was consistent between patients with and without evidence of allergic inflammation and the maximum effect was achieved by Week 12 and sustained to Week 52.20These results are supported by a previous post hoc analysis, in which similar results were observed in QUEST patients with and without evidence of allergic asthma.21
Dupilumab Efficacy in Patients with Uncontrolled, Moderate-to-Severe Asthma by Immunoglobulin E Levels at Baseline
Doctor Warner W. Carr
This post hoc analysis assessed the effect of dupilumab on severe exacerbations and FEV1, as well as the impact on overall asthma control in patients with uncontrolled, moderate-to-severe asthma as defined by baseline IgE levels. The aim was to investigate whether there was a differential effect on these efficacy measures defined by baseline IgE levels. The study assessments included the annualised rate of severe exacerbations, LS mean change from baseline in pre-BD FEV1(L), and LS mean change from baseline in the 5-item Asthma Control Questionnaire (ACQ-5) score during the 52-week treatment period.22
Patients with uncontrolled, moderate-to-severe asthma were characterised at baseline by IgE level (381 patients had an IgE level <100; 782 patients had ≥100 to <500; 419 patients had ≥500; 313 patients had ≥700; and 212 patients had ≥1,000 IU/mL Figure 1. Baseline demographics and disease characteristics were generally similar across IgE groups.22
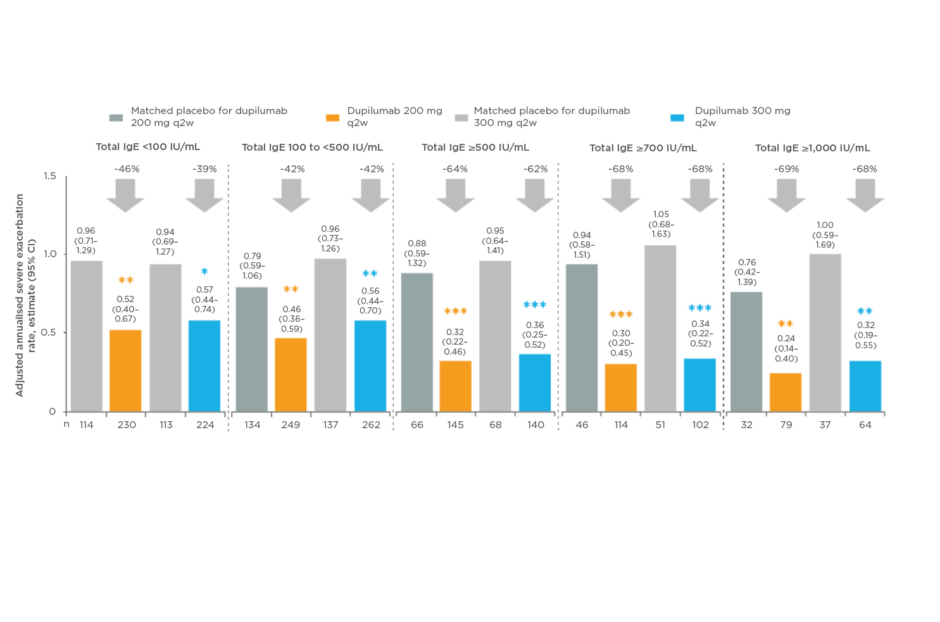
Figure 1: Dupilumab significantly reduced severe exacerbations in all baseline IgE groups.22
*p<0.05; **p<0.01; ***p<0.001 versus matched placebo.
CI: confidence interval; IgE: immunoglobulin-E.
Dupilumab 200 mg and 300 mg every 2 weeks versus placebo significantly reduced severe exacerbations in all baseline IgE groups (-38.9 to -67.9%; all p<0.05) and significantly improved pre-BD FEV1at Weeks 24 and 52 in all baseline IgE groups (LS mean difference: 0.11–0.31 L; all p<0.05), except for 300 mg in IgE <100 IU/mL and ≥1,000 IU/mL groups (Figure 2).22
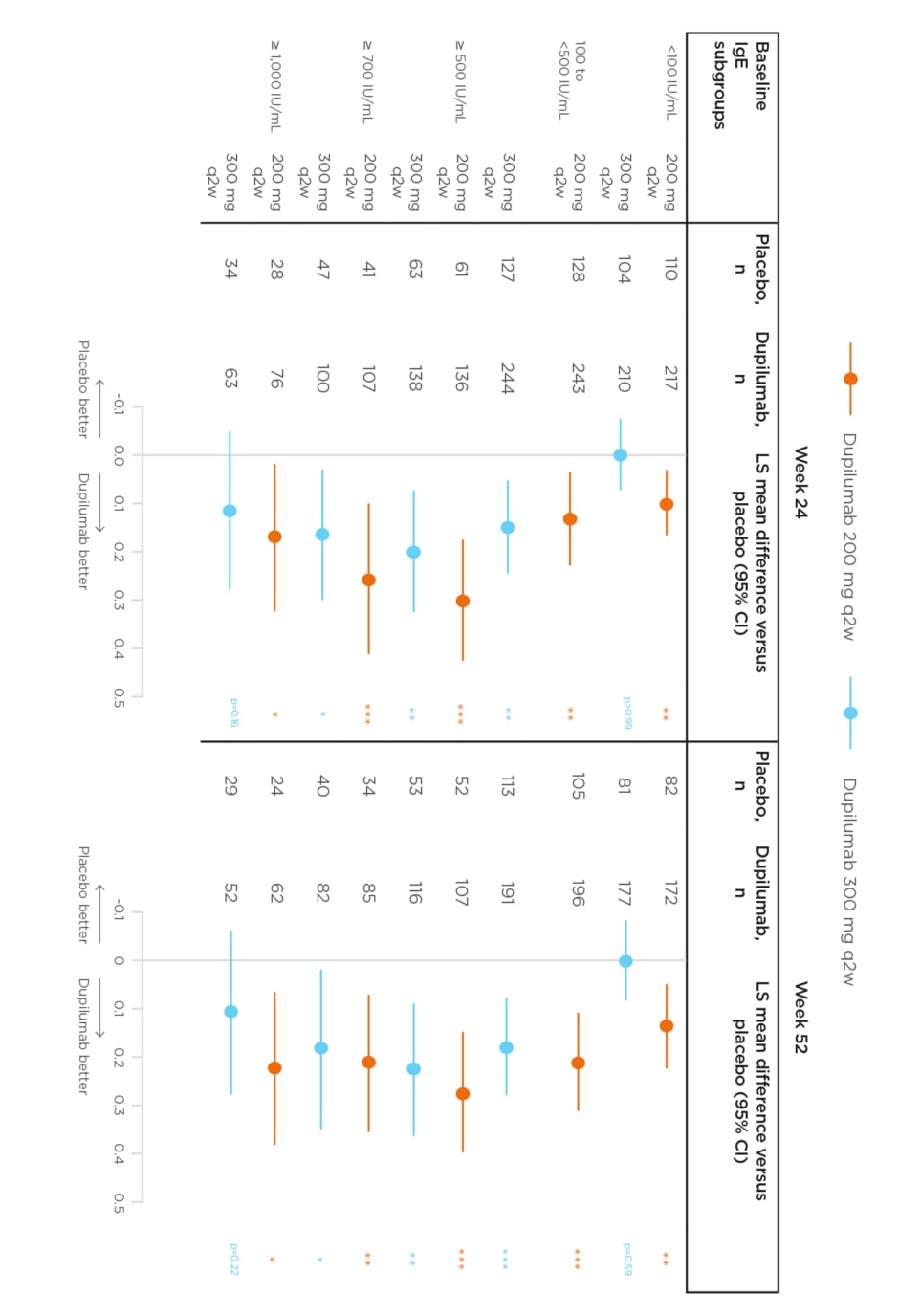
Figure 2: Effect of dupilumab on the change from baseline in least squares mean pre-bronchodilator forced expiratory volume in 1 second by IgE levels at Weeks 24 and 52.22
*p<0.05; **p<0.01; ***p<0.001 versus matched placebo.
CI: confidence interval; IgE: immunoglobulin-E; LS: least squares; q2w: every 2 weeks.
In the overall safety population, the incidence of TEAE was similar across treatment groups. Conjunctivitis was observed in 2.3% versus 3.3% of patients receiving dupilumab versus placebo, respectively.22
Dr Carr concluded that in general, baseline demographics and disease characteristics were balanced between treatment groups across the patient subgroups by total serum IgE levels at baseline. Dupilumab reduced severe asthma exacerbation rates and improved FEV1and asthma control in patients with moderate-to-severe asthma in all IgE subgroups. For exacerbations, these effects reached statistical significance for both dupilumab 200 mg and 300 mg every 2 weeks groups in all IgE subgroups. For FEV1and asthma control, these effects reached statistical significance for patients receiving dupilumab 200 mg every 2 weeks in all IgE subgroups; but for where dupilumab was prescribed 300 mg every 2 weeks dose, not all IgE subgroups reached statistical significance. In conclusion, regardless of atopic status as categorised by baseline IgE levels, dupilumab can reduce severe exacerbations and improve FEV1.22
Dupilumab Efficacy in Type 2 Inflammatory Asthma: Liberty Asthma QUEST Study (Poster OA3807)
Professor Ian D. Pavord
The new GINA report for difficult-to-treat and severe asthma proposes baseline blood Eos ≥150 cells/µL and/or baseline FeNO ≥20 parts per billion (ppb) as cut-offs to define Type 2 inflammatory asthma.23
This post hoc analysis assessed dupilumab efficacy in patient subgroups defined by the GINA proposed markers for Type 2 asthma, namely in patients with baseline Eos ≥150 cells/µL, FeNO ≥20 ppb, and in other quadrant subgroups. The endpoints assessed included annualised rate of severe exacerbations during the 52-week treatment period and change from baseline in pre-BD FEV1(L) at Week 12.24
Baseline disease characteristics were generally comparable across the subgroups. The mean age was 47.20 years, 59.00% were female, the mean percent predicted pre-BD FEV1was 58.55, the mean exacerbations in the past year was 2.22, and the mean ACQ-5 score was 2.76.24
In patients with baseline Eos ≥150 cells/µL and FeNO ≥20 ppb (n=922), dupilumab 200 mg and 300 mg every 2 weeks versus placebo significantly reduced severe exacerbations by 66% and 63% respectively, and improved FEV1 by 0.26 L and 0.22 L, respectively (all p<0.0001). Similar results were observed at Week 52 and dupilumab efficacy was not significant in the other patient subgroups.24
Overall, the most frequently reported AE in the dupilumab versus placebo group was injection-site reactions.24
Prof Pavord concluded that dupilumab significantly reduced severe exacerbations and improved FEV1in patients with Type 2 inflammatory asthma. Moreover, the effect of dupilumab treatment in reducing exacerbations and improving FEV1was greatest in patients with elevation of both baseline blood EoS count (≥150 cells/µL) and FeNO (≥20 ppb).24
Dupilumab Efficacy in Asthma Patients with FEV160–80% Predicted on Medium-Dose Inhaled Corticosteroids : LIBERTY ASTHMA QUEST Study
Professor Alberto Papi
This post hoc analysis aimed to assess dupilumab efficacy in patients with moderate asthma defined as asthma with baseline pre-BD FEV160–80% predicted (60–90% in adolescents <18 years), on medium-dose ICS (implying milder asthma than other QUEST patients), and one or more additional controller therapy, without a minimum requirement for baseline blood Eos count or FeNO. The co-primary endpoints were the annualised severe asthma exacerbation rates during the 52-week treatment period and the change from baseline in pre-BD FEV1at Week 12, analysed using negative binomial models and mixed-effects models with repeated measures, respectively. Study assessments included the annualised severe exacerbation rates, LS mean change from baseline in pre-BD FEV1(L), and LS mean change from baseline in the ACQ-5 score during the 52-week treatment period. The medium ICS dose was fluticasone propionate at a total daily dose of 250–500 μg or an equipotent equivalent.25
Twenty-seven percent (517/1,902) of patients had pre-BD FEV160–80% predicted and were on medium-dose ICS at baseline. The mean age was 43.50 years, 61.90% were female, the mean percent predicted pre-BD FEV1was 69.49, the mean exacerbations in the past year was 1.82, and the mean ACQ-5 score was 2.56.25
In these patients, dupilumab 200 mg and 300 mg every 2 weeks versus placebo reduced annualised severe exacerbation rates by 44% and 51%, respectively (p=0.06; p=0.01; Figure 3.
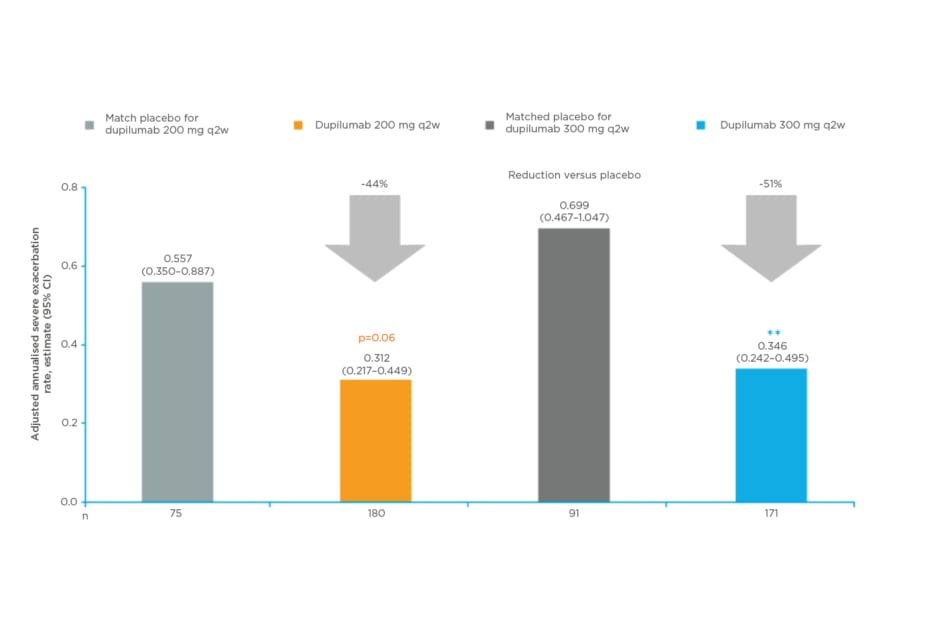
Figure 3: Dupilumab reduced severe exacerbations in patients with baseline pre-bronchodilator forced expiratory volume in 1 second 60–80% predicted (or 60–90% in adolescents <18 years) on medium-dose inhaled corticosteroids .25
**p<0.01 versus matched placebo.
Dupilumab 200 mg and 300 mg q2w reduced annualised severe exacerbation rates versus placebo. The annualised rates of severe exacerbations during the 52-week treatment period were analysed using negative binomial regression models.
CI: confidence interval; q2w: every 2 weeks.
Dupilumab 200 mg and 300 mg every 2 weeks versus placebo also improved FEV1at Week 12 with a LS mean difference of 0.11 L/ 0.09 L, respectively (p=0.01/p=0.05).25
Overall, the most frequent adverse event reported in the dupilumab 200 mg and 300 mg groups versus placebo groups were injection-site reactions (15%/18% versus 5%/10%).25
Conclusion
Prof Papi concluded that dupilumab demonstrated meaningful reductions in severe exacerbations and significantly improved pre-BD FEV1in the studied patient population. The magnitude of these effects was comparable to those previously seen in the LIBERTY ASTHMA QUEST patients with severe asthma. Numerical improvements in ACQ-5 were observed at all time points, with the trend comparable to results observed in the overall QUEST population and, furthermore, dupilumab was generally well-tolerated.25
Dupilumab is approved in the European Union (EU) for patients >12 years as an add-on maintenance treatment for severe asthma with Type 2 inflammation. This is characterised by raised blood EoS and/or raised FeNO; inadequately controlled with high dose ICS and another medicinal product for maintenance treatment; and in certain patients with asthma, chronic rhinosinusitis with nasal polyps, or atopic dermatitis in a number of countries.17,26-33These four posters presented at the ERS International Congress 2019 demonstrate that dupilumab treatment is relatively well-tolerated and could significantly improve FEV1, symptoms, asthma control, quality of life, and reduce severe exacerbation risk in patients with uncontrolled asthma. This therapy offers an important new option for respiratory clinicians to manage their patients with uncontrolled asthma.








