Meeting Summary
The main objectives of the two symposia were to raise awareness of nontuberculous mycobacterial lung disease (NTM-LD); evaluate the key microbiological and clinical aspects of the disease, including its association with other conditions, such as bronchiectasis and common coinfections; outline the current treatment and management strategies; and review data from clinical trials of new therapies and how these could shape future management strategies. Dr Chalmers, Dr Griffith, and Dr Haworth opened the symposia by introducing NTM-LD and providing a brief overview of the key topics. Dr Ringshausen focussed on the epidemiology, prevalence, and burden of NTM-LD, and briefly discussed pathophysiology. Dr van Ingen outlined the microbiological diagnosis of NTM-LD, in particular the importance of molecular identification and drug susceptibility testing (DST). Dr Aliberti introduced bronchiectasis, outlined the relationship between the two diseases, and discussed the clinical relevance of comorbid disease. Dr Aksamit addressed the assessment and management of co-isolated NTM and other respiratory pathogens. Dr Griffith and Dr Loebinger each summarised the current treatment and management strategies, and reviewed the latest research regarding new therapies and what this could mean for the future. Dr Koh closed the symposium by outlining the latest clinical research on the natural history of NTM-LD from a global perspective.
Prevalence and Burden: Why Care About Nontuberculous Mycobacterial Lung Disease?
Doctor Felix Ringshausen
The epidemiology of NTM-LD is difficult to predict, as it is not a reportable disease and surveillance systems do not exist. One initiative in the Netherlands gathered data from >20,000 laboratory isolates from 30 different countries and found that Mycobacterium avium complex (MAC) was the most frequently isolated NTM (47% of isolates), although the predominant species and strain varied between countries.1 A retrospective cohort of NTM-LD over 25 years in Denmark demonstrated no increase in disease burden or incidence, and identified children aged 0–4 years as having the highest incidence of NTM disease.2 In contrast, a study of burden and trends of NTM-LD-associated hospitalisations in Germany showed a significant increase in incidence from 2005–2011. Chronic obstructive pulmonary disease (COPD) was identified as the most frequent NTM-LD-associated condition (28.9% of hospitalisations), and the average annual increase in this patient population was 4.8% (Figure 1).3 Likewise, in a USA study of insurance claims data from 1997–2007, the prevalence of NTM-LD increased annually by 8.2%.4 A similar study in Germany that used health insurance claims data showed that the prevalence of NTM-LD increased from 2.3 to 3.3 cases/100,000 population from 2009–2014 and that >50% of patients had concomitant COPD. The highest prevalence rates were among patients aged ≥80 years, with a rate of 9.4 and 9.6/100,000 for men and women, respectively.5
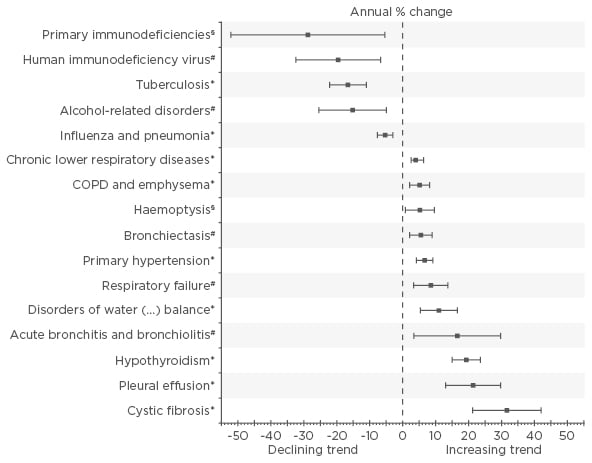
Figure 1: Average annual percentage change of the rate of associated primary and secondary diagnoses per 1,000 hospitalised patients with any diagnosis of nontuberculous mycobacterium lung disease.
Bars indicate 95% confidence intervals calculated from Poisson log-linear regression (Wald statistics). Non-significant trends from 2005–2011 are not shown.
*Statistical significance at p<0.001; #Statistical significance at p<0.01; §Statistical significance at p<0.05.
COPD: chronic obstructive pulmonary disease.
Adapted from Ringshausen et al.3
To explore the burden of NTM-LD in Germany, Diel et al.6 carried out an analysis of 125 patients with NTM-LD, compared with 1,250 matched control patients. The incidence rate of NTM-LD was 2.6/100,000. The mean direct expenditure per patient with NTM-LD was four-fold that of matched controls (€39,559 versus €10,006), while hospitalisations were three times higher in patients with NTM-LD compared with matched controls and accounted for 63% of total costs. Attributable annual direct costs and indirect work- loss costs in patients with NTM-LD were €9,093 and €1,221 per patient, respectively. It was also found that 74% of patients with NTM-LD received antibiotics (29 different regimens were prescribed) and 12% were prescribed macrolide monotherapy. The mortality rate in patients with NTM-LD was higher than in matched controls, 22.4% and 6.0%, respectively, which was increased with concomitant COPD (41.5%).
Pathogenesis of Nontuberculous Mycobacterium Lung Disease
The pathogenesis of NTM-LD differs according to the pathogen, with species, subspecies, virulence/resistance mechanisms, and interactions with other pathogens all influencing factors. Environmental factors, such as temperature, evapotranspiration, pH, water chlorine content, biofilms, and soil mineral matter, also play a role, along with host factors, such as the presence of structural or genetic immunological diseases and certain phenotypes.
Microbiological Diagnosis of Nontuberculous Mycobacterium Lung Disease
Doctor Jakko van Ingen
Laboratory diagnosis of NTM-LD should involve mycobacterial culture on liquid and solid media at 30°C and 37°C, respectively, and partnership with a reference lab should allow culture with supplemented media, molecular identification of NTM to species level, and guideline-compliant drug susceptibility testing (DST). Molecular detection of resistance-conferring mutations and next-generation sequencing of NTM in clinical specimens should be on the research agenda. The clinical relevance of NTM isolates differs by species, so correct species identification is crucial to allow treatment to be evidence-based.
Although there are no validated commercial assays, a study comparing the correlation between polymerase chain reaction (PCR) and culture demonstrated that in smear-positive specimens, PCR detection of NTM had 100% sensitivity and 97% specificity, while in smear-negative specimens it had 50% sensitivity and 90% specificity.7 Identification of isolated NTM using molecular methods is important for rapid diagnosis and treatment decisions. Fibrocavitary disease (FCD) is easier to diagnose microbiologically, as sputum cultures are often positive and direct Ziehl–Neelsen staining of sputum often reveals acid-fast bacilli (AFB). Nodular bronchiectatic (NB)-LD is harder to diagnose as it has a lower bacterial burden and the sensitivity of direct staining, PCR, and culture is lower. A series of sputum samples or even a bronchoalveolar lavage sample may be required to find the causative NTM and meet the microbiological criteria for NTM-LD diagnosis.
Drug Susceptibility Testing
There is a myth that for NTM, results of in vitro DST do not correlate with outcomes of treatment in vivo.8-14 There is, however, sufficient evidence from clinical studies that susceptibility to macrolides and amikacin is correlated to outcomes of treatment. For rifampicin and ethambutol, which are prescribed for their synergy and not their individual effects, the activity against MAC in vitro is not important and should not be reported by laboratories.
Overall, it is important to have a multidisciplinary team to treat NTM-LD, including a pharmacist and a microbiology team who can identify to the species level and partner with reference laboratories. Quality control should be stringent and DST should be carried out according to existing guidelines.
Bronchiectasis as a Friend of Nontuberculous Mycobacterium and Vice Versa
Doctor Stefano Aliberti
Bronchiectasis is a disease with an increasing prevalence and incidence worldwide.15 Historically, it has been neglected, which has contributed to a lack of awareness and licenced treatments, with guidelines recommending reliance on real-life experience to manage the disease. Symptoms of bronchiectasis include chronic cough, haemoptysis, purulent sputum, wheeze, tiredness, and recurrent lower respiratory tract infections. However, it is a highly heterogeneous disease with variable aetiology, radiological findings, and pulmonary function. Chronic infection plays a crucial role in the vicious cycle of bronchiectasis pathophysiology; infection increases inflammation and leads to airway damage and development of bronchiectasis, which impairs lung defences and leaves the patient vulnerable to chronic infection.16
In 2016, the European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC)released a statement outlining research priorities in bronchiectasis, the third highest priority being the prevalence and characteristics of microbiological colonisation and chronic and acute infection (including NTM) in patients across Europe.17 The European Bronchiectasis Registry enrolled 9,123 patients from 27 countries and found that <5% of patients were infected with NTM.18
Nontuberculous Mycobacterium and Bronchiectasis: A Specific Clinical Phenotype
Among patients with bronchiectasis, there is a clear phenotype for those that have NTM: elderly (>60 years of age), female, with a low BMI, scoliosis, mitral valve prolapse, and no pre-existing LD. An interesting association exists between NTM-LD and cystic fibrosis transmembrane conductance regulator (CFTR) mutation. The clinical course is a prolonged cough with fatigue and weight loss, and radiology showing bronchiectasis with nodules particularly in the middle and upper lobes.
Prevalence of Nontuberculous Mycobacterium in Bronchiectasis
The prevalence of NTM in bronchiectasis varies between countries and study type (Table 1), and evaluation of the data is limited by low sample size, variable study periods, geographic locations, and the fact that most of the data are from case series, retrospective analyses, or databases.
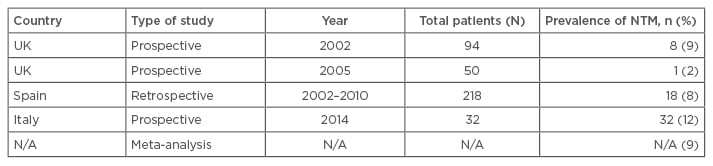
Table 1: Prevalence of nontuberculous mycobacterium in bronchiectasis.19-24
N/A: not applicable; NTM: nontuberculous mycobacteria.
Sputum Culture for Mycobacteria in Bronchiectasis
The Italian national audit of patients with bronchiectasis across 32 hospitals during 2014–2015 enrolled 1,361 patients (53% female; average age 70 years) and found that sputum culture during the stable period was performed in <30% of patients, and only 50% of cultures were for mycobacteria.25 In a prospective, observational, single-centre study from 2012–2015, 261 adults with bronchiectasis were identified (59% female; average age 69 years), of which 52% underwent bronchoscopy, 12% had NTM-LD (59% identified by fetal bovine serum and 41% from sputum), 4% had coinfection with Pseudomonas aeruginosa, and none were resistant to macrolides. Patients with NTM-LD were more likely to have cylindrical bronchiectasis (p=0.048), a ‘tree-in-bud’ pattern (p=0.011) on radiology, and a history of weight loss (p=0.045) compared with those infected with P. aeruginosa.23
Additionally, NTM-LD in bronchiectasis can be complicated by coinfection with other bacteria (such as P. aeruginosa), and fungi (such as Aspergillus).19,26 The presence of CFTR gene polymorphisms without cystic fibrosis (CF) may predispose to NTM infection by increasing the risk of bronchiectasis.27
American Thoracic Society Diagnostic Criteria
The American Thoracic Society (ATS) guidelines recommend that the following diagnostic criteria are met for diagnosis of NTM-LD: symptoms compatible with NTM-LD, radiology compatible with NTM-LD (e.g. cavities, nodules), ≥3 respiratory samples over ≥1 week, and ≥2 positive cultures with the same species (identified on both solid and liquid media plus molecular methods). For patients with bronchiectasis, the diagnosis of NTM-LD is very difficult as they already have the clinical and radiological criteria due to bronchiectasis.28
In conclusion, the diagnosis of NTM in patients with pre-existing bronchiectasis can be challenging. Cultures should be taken before macrolide therapy is initiated, and resistance should be monitored.
How to Assess and Manage the Co-Isolation of Nontuberculous Mycobacteria with Other Potential Respiratory Pathogens
Doctor Timothy Aksamit
The isolation of Mycobacterium tuberculosis and NTM is common, and the presence of multiple organisms is not rare. As a general rule, tuberculosis (TB) should be treated first and then NTM-LD reassessed. Notably, not all smear-positive disease is NTM (in the USA only 14% of smear-positive isolates were NTM).29 A recent report supported the recommendation to restrict investigations to sputum smear-positive patients;30 however, NTM should be considered when TB is being investigated. For example, in a cohort of patients with multiple drug-resistant TB in Cambodia, 409 patients were identified, of whom 46% were examined for culture. Two-thirds of the samples were culture-positive; 25% for NTM and 75% for M. tuberculosis.31
It is very common to isolate multiple NTM in one sample. For example, in a retrospective National Jewish Group study of 107 patients with NTM-LD, over half (55%) of patients had coexistent or previous MAC infection,13 whereas in another study of patients with CF, the initial isolate was almost always a single organism.32
A study of patients with MAC lung disease (MAC-LD) explored the significance of Mycobacterium abscessus subspecies abscessus (MAA) infection. In total, 29% of patients had MAA infection and the time from MAC-LD diagnosis to first MAA was between 9.9 and 11.3 months. Patients with MAA infections were more likely to have new or enlarging cavitary lesions on MAC therapy than those without coinfection (38% versus 0%, respectively).33 Treatment for MAC and MAA coinfection should include ethambutol, rifampin, a macrolide, amikacin, cefoxitin, and imipenem.
Aspergillus is a fungus that is more common in patients with NTM-LD than those without; the US Bronchiectasis Research Registry demonstrated that Aspergillus was present in 21% of patients with NTM-LD, versus 16% without.33 A cohort of patients with CF showed that 30% versus 8% of patients with and without NTM-LD, respectively, also had Aspergillus infection.32 Similarly, a retrospective study that compared 30 patients with NTM-LD and 61 patients with bronchiectasis without NTM-LD found that Aspergillus was present in 20% versus 0% of patients with and without NTM-LD, respectively.26 In the ATS Fungal Statement in 2011, voriconazole, itraconazole, posaconazole, amphotericin, and caspofungin were identified as treatments for Aspergillus infection;34 however, drug–drug interactions between NTM-LD and Aspergillus therapies limit the number of options. Although treatment with dose-adjusted antifungals and rifabutin has been carried out, along with therapeutic drug monitoring,35 it is not generally advised. Other options are to delay NTM treatment until Aspergillus infection is cured or to use alternative strategies that exclude rifamycin/rifabutin.
In conclusion, coinfection in NTM-LD is not uncommon, although the impact on natural history is yet to be established. Careful screening and regular monitoring for other pathogens is crucial for the care of patients with NTM-LD. Complex multidrug regimens that bear several drug–drug interactions are the only options currently available, and therapeutic drug monitoring should be utilised when treatment is prescribed.
Management of Nontuberculous Mycobacterium Treatment and New Strategies
Doctor David Griffith and Doctor Michael Loebinger
There are several factors that should be considered when making the decision whether to treat NTM-LD or not, such as whether NTM-LD is associated with cavities, the symptoms of the patient, pulmonary comorbidities, short and long-term prognosis, and the patient’s wishes. Additionally, at present there are a number of impediments to effective NTM-LD therapy, such as the long duration of therapy required, multiple drug cocktails, ongoing exposure to NTM from the environment, innate and acquired antibiotic resistance, poor clinical response to drugs, and patient factors (such as comorbid conditions).
Nontuberculous Mycobacterium Drug Resistance
Drug resistance can be either innate, i.e. natural drug resistance that is not readily associated with in vitro measures of resistance, such as minimal inhibitory concentrations (MIC) (for example, inducible macrolide resistance through the erm gene) or acquired, where isolates are selected with naturally occurring mutations that confer resistance to specific antibiotics. Some species of NTM, such as M. abscessus, MAC, and Mycobacterium simiae, show ‘cryptic’ resistance, whereby in vitro susceptibility results do not correlate with in vivo treatment response. However, some species, such as Mycobacterium kansasii (rmp), Mycobacterium marinum, Mycobacterium szulgai, and Mycobacterium fortuitum (erm), show good correlations between in vitro susceptibility and treatment response.
Treatment of Nontuberculous Mycobacterium
It is recommended to carry out initial in vitro susceptibility testing for NTM isolates to guide therapy, for example, to test the effectiveness of macrolides and amikacin against MAC. Gene mutations that confer resistance among NTM include the 23S rRNA gene and 16S rRNA gene in MAC, which confer resistance to macrolides and amikacin, respectively, the rpoβ gene in M. kansasii, which confers resistance to rifamycins, and the 23S rRNA gene in MAA/M. abscessus subspecies massiliense (MAM), which confers resistance to macrolides. Inadequate therapy in such cases results in acquired mutational resistance and can cause profound negative consequences for the treatment of NTM-LD.
In a study of 71 patients with M. massiliense LD, patients received oral macrolides along with 4 or 2 weeks initial intravenous (IV) amikacin and cefoxitin (or imipenem). Patients were treated for 24 months (4-week IV group) or for ≥12 months after negative sputum culture conversion (2-week IV group). Response rates after 12 months were 89% versus 100% for symptoms, 79% versus 91% for computed tomography (CT) scanning, and 100% versus 91% for negative sputum culture results in the 4 and 2-week IV groups, respectively. Acquired macrolide resistance developed in 2 patients in the 2-week IV group. Genotyping analysis for patients who failed to convert sputum culture to negative, or those with positive culture after successful treatment, revealed that most episodes were due to reinfection with different genotypes of M. massiliense.36 In an editorial by Griffith and Aksamit,37 the authors outlined that M. massiliense without an active erm gene is responsive to macrolide treatment, and that the primary mechanism of macrolide resistance is acquired mutational resistance. This is noteworthy as it is inevitable that some patients treated with macrolide monotherapy will develop resistance and become treatment-refractory.
The pharmacokinetic and pharmacodynamic indices of MAC therapy are often suboptimal, although currently there is no correlation with treatment outcome and no demonstrated correlation between circulating MAC drug levels and treatment outcome. There is also no correlation between MIC for rmp/emb/stm and response to therapy and no MIC cut-offs designating susceptible and resistant MAC strains for any antibiotics other than macrolides and amikacin. Additionally, inappropriate dependence on in vitro susceptibility results may have adverse consequences. Macrolides and amikacin treatment success for MAC correlates with in vitro MIC; with macrolides the susceptible MIC is ≤8 μg/mL and resistance MIC is ≥32 μg/mL, and with amikacin the susceptible MIC is ≤64 μg/mL and resistance MIC is >64 μg/mL.
The ATS guidelines on NTM recommend the following for NTM-LD for ≥12 months with sputum culture negativity on therapy:28
- NB disease: macrolide/ethambutol/ rifamycin; intermittent
- Cavitary disease: macrolide/EMB/ rifamycin ± injectable; daily
- Severe or previously treated disease: macrolide/EMB/rifamycin/injectable; daily
- Surgery for selected patients
Current treatment strategies for MAC-LD include a combination of rifampicin, ethambutol, azithromycin, and clarithromycin with the addition of IV amikacin or ≤3 months nebulised amikacin for severe cases. Antibiotic treatment should continue for ≥12 months after culture conversion (unpublished data). Treatment of M. abscessus LD should involve an initial phase of ≥1 month of IV amikacin, IV tigecycline, and, where tolerated, IV imipenem plus oral macrolide followed by a continuation phase of nebulised amikacin, oral macrolide, and 1–3 oral antibiotics guided by drug susceptibility and patient tolerance. Despite use of these regimens, mortality remains high, with a 5-year all-cause mortality reported between 23–70% in a variety of retrospective cohort studies of different mycobacterial species.38-41 In addition to the high mortality rates, significant numbers of patients remain refractory with continued isolation of mycobacteria. The highest culture conversion success rates are generally with M. kansasii, and the worst outcomes for Mycobacterium xenopi or M. abscessus. A recent meta-analysis of 16 studies and 1,462 patients suggested a sustained conversion rate of 60% in MAC-LD.42,43
A retrospective single-centre study aimed to validate the appropriateness of current treatment for MAC-LD. In total, 180 patients with NB-LD completed >12 months of macrolide/azalide multidrug therapy. Sputum conversion to culture-negativity occurred in 86% of patients, with no differences between clarithromycin or azithromycin regimens. Treatment regimen modification occurred more frequently with daily (80%) versus intermittent (1%) therapy. Microbiological recurrences during therapy occurred in 14% of patients: 73% with reinfection MAC isolates and 27% with true relapse isolates. Overall treatment success was achieved in 84% of patients. Following completion of therapy, microbiological recurrences occurred in 48%: 75% reinfection isolates and 25% true relapse isolates.44 A similar study in South Korea of 217 patients with NB MAC-LD found that rates of symptomatic improvement, radiographic improvement, and sputum culture conversion were not different between daily therapy and intermittent therapy (75% versus 82%, 68% versus 73%, and 76% versus 67%, respectively). Modification of the initial antibiotic regimen occurred more frequently in the daily therapy group than in the intermittent therapy group (46% versus 21%).45
Overall, current guidelines for macrolide/azalide-based regimens for NB MAC-LD show favourable microbiological outcomes and do not promote macrolide resistance. Daily and intermittent regimens are equally effective, although intermittent regimens have fewer side effects. Microbiological recurrences are common and mostly due to unique MAC genotypes. However, there is a lack of adherence to guidelines in the USA, Europe, and Japan.46,47
Cavitary Mycobacterium Avium Complex Nontuberculous Mycobacterium Lung Disease
Cavitary MAC NTM-LD is a smoking COPD-related disease that is likely associated with long-term respiratory impairment and requires aggressive therapy. Appropriate therapy includes parenteral agents, surgery, smoking cessation, and avoidance of macrolide resistance. Patients should be advised that smoking during treatment can inhibit a favourable treatment response.
A National Institutes of Health (NIH) study of mortality among patients with NTM-LD demonstrated that FCD and pulmonary hypertension (PH) were significant risk factors for death in NTM-LD. Median survival for patients with FCD was 9.0 years versus 13.1 years with no FCD (p=0.006), and 6.8 years for patients with PH versus >18 years for those without PH (p=0.048).48
Surgery for Nontuberculous Mycobacterium Lung Disease
Surgery is indicated for NTM-LD if medication is unresponsive (due to resistance or large cavities) or if the patient has uncontrolled symptoms, haemoptysis, or highly damaged lung tissue.
The Nontuberculous Mycobacterium Lung Disease Drug Pipeline
New drugs for NTM-LD should be tested using sputum conversion, duration of therapy, and symptomatic improvement as endpoints. Currently, liposomal amikacin inhalation (LAI) is under investigation for the treatment of NTM-LD and the U.S. Food and Drug Administration (FDA)-approved drugs (bedaquiline, linezolid, tedizolid, clofazimine) are being studied for potential use.
There is some evidence that a proportion of patients with stable NTM-LD do not require treatment and culture-convert spontaneously. In an observational study by Hwang et al.,49 93 patients (out of 420) with MAC-LD were untreated and followed up for a median of 5.6 years. Of these patients, 51.6% showed spontaneous sputum conversion.
Nebulised Amikacin
A study of nebulised amikacin in 20 patients with MAC or M. abscessus suggested some response to therapy, with 5 patients demonstrating negative cultures after treatment.50
Liposomal Amikacin Inhalation
In a Phase II study, the efficacy and safety of LAI 590 mg once-daily versus placebo was assessed for 84 days with an additional open-label treatment period of 84 days. The primary endpoint was reduction in NTM growth by semi-quantitative sputum cultures. The primary endpoint was not achieved, although a greater proportion of patients in the LAI group demonstrated ≥1 negative sputum culture (32% versus 9%; p=0.006) and improvement in 6-minute-walk test at Day 84. Most adverse events were respiratory (hoarseness and bronchospasm) and led to drug discontinuation in some patients. Of those who achieved culture conversion after 3–6 months of LAI, 82.4% had negative sputum culture at 12 months after LAI discontinuation.11
The Phase III CONVERT study of LAI therapy enrolled 336 patients with NTM-LD caused by MAC who were treatment refractory for ≥6 months of guideline-based therapy (GBT). Top line results have been released that show the primary endpoint, culture conversion, was met (29% LAI + GBT versus 9% GBT-placebo; p<0.0001).51
Bedaquiline
Bedaquiline is a drug used to treat TB and has been investigated for potential use in NTM-LD. A study of untreated MAC isolates MIC90 with bedaquiline 0.004–0.008 μg/mL using Clinical and Laboratory Standards Institute methods demonstrated no difference between M. avium and Mycobacterium intracellulare.52 Preliminary results of an open case series of off-label bedaquiline use in 10 patients with MAC or M. abscessus LD showed some symptomatic benefit; however, microbiological and radiological outcomes were mixed.53
Linezolid and Tedizolid
Linezolid is indicated in the British Thoracic Society (BTS) guidelines for possible use against M. abscessus disease, although clinical evidence of efficacy is lacking. One study assessed the tolerability of linezolid for NTM-LD; cytopenia, peripheral neuropathy, and optic neuropathy were identified as significant adverse events.54
In a further study, the potency of tedizolid was compared with linezolid using in vitro testing of 170 NTM isolates, including resistant strains. Tedizolid demonstrated a greater in vitro potency compared with linezolid (Table 2), which indicated its use as a potential treatment for NTM infections.55
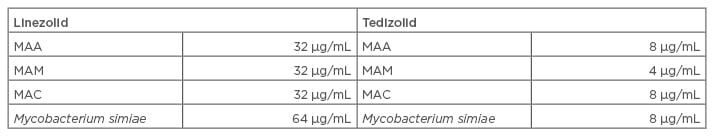
Table 2: In vitro susceptibility results of tedizolid against nontuberculous mycobacterium.55
MAA: Mycobacterium abscessus subspecies abscessus; MAC: Mycobacterium avium complex; MAM: M. abscessus subspecies massiliense.
Clofazimine-containing regimens have been shown to be effective when compared with rifampin-containing regimens for the treatment of MAC-LD in a study of 107 patients and may be a suitable alternative.56
Other Strategies
In addition to new drugs, different treatment paradigms may be needed to improve treatment success. Presently, treatment is advised for 1 year following culture conversion. Despite apparent treatment success, high relapse and reinfection rates ≤48% have been reported.44 It is possible that longer treatment periods may be needed for patients with higher relapse rates (such as those with cavitary disease) or prophylactic treatment post eradication may reduce the risk of reinfection. NTM are ubiquitous in the environment, so preventing exposure is challenging; however, certain cleaning regimens may be more effective at environmental eradication of NTM.
An increase in precision medicine and targeting specific treatments to certain individuals is likely to occur in the future. For example, patients with specific predispositions or susceptibilities to NTM-LD may benefit from a treatment approach that addresses the host in addition to the microbe, such as with the use of immunomodulators. Additionally, as we learn more about the microbes with technologies such as sequencing, treatments directly affecting virulence targets, biofilms, and the microbiome are likely to develop.
The Natural History of Untreated Nontuberculous Mycobacterium Lung Disease: A Global Perspective
Doctor Won-Jung Koh
The natural history of untreated pulmonary TB was studied in 126 AFB smear-positive patients in South India from 1961–1968 when no treatment was available. At the end of the study, 49% of patients had died, 18% had chronic disease, and 33% were cured without treatment.57 This study was not powered to detect NTM; however, more recent studies have provided better understanding of the natural history of NTM-LD.
MAC (M. avium and M. intracellulare) and M. abscessus complex (MABC) (M. abscessus and M. massiliense) are the most common causes of NTM-LD (approximately 90% of cases); therefore, natural history studies have focussed on these four pathogens. Fibrocavitary NTM-LD is typically caused by M. intracellulare and M. massiliense and patients are generally middle-aged/elderly males with underlying pulmonary disorders such as COPD and TB. Chest X-ray and CT scans show thin-walled apical cavity in the upper lobes of the lung, which, if left untreated, can progress in 1–2 years to cause extensive lung damage and death. NB NTM-LD is typically caused by M. intracellulare and patients are generally middle-aged/elderly females without underlying pulmonary disorders, who do not smoke. A high-resolution CT scan shows multiple small nodules (<5 mm) and bronchiectasis in both lungs, and the course of disease is indolent with slow progression.
Mycobacterium Avium Complex Lung Disease Mortality
In a retrospective study of 634 Japanese patients with MAC-LD, 76%, 21%, and 3% had NB-LD, fibrocavitary LD, or other forms of NTM-LD, respectively. Kaplan-Meier curves of all-cause mortality according to radiographic features showed that the survival rate of patients with FCD was significantly lower than those with NB-LD or other forms of NTM-LD (p<0.001).40 In a similar study of 782 patients with NB MAC-LD, 85% had non-cavitary disease and 15% of patients had cavitary disease. Ten-year survival rates were significantly lower for patients with cavitary disease (25%) compared with those without cavitary disease (0.8%); (p<0.001).58 Taken together, these results demonstrate that cavitation is an important prognostic factor in both FCD and NB-LD.
Nontuberculous Mycobacterium Lung Disease Treatment Decision
The ATS guidelines state that a diagnosis of NTM-LD does not necessarily lead to the initiation of treatment; they recommend that the decision to initiate treatment for patients with NTM-LD should be based on risk-to-benefit analysis that takes into account patient symptoms, radiographic findings (such as severity and progression), and microbiological results versus the adverse effects of multiple potentially toxic drugs.28 In clinical practice, the proportion of patients who receive treatment for NTM-LD varies according to the country: population-based studies in the USA, the Netherlands, and Germany found that 19%, 57%, and 75% of patients received treatment, respectively.6,59,60
Mycobacterium Avium Complex Lung Disease and Mycobacterium Abscessus Complex Lung Disease Progression
Hospital-based studies evaluating progression of MAC-LD (defined as initiation of therapy) showed that 43% of 392 patients in the USA were started on treatment,61 53% of 72 patients with NB-LD in Japan showed progressive disease on chest X-ray at 10 years follow-up,62 and 51% of 67 patients with NB-LD in South Korea had progressive disease at 5-year follow-up.63 In these patients, risk factors for progression included low BMI, cavitation, and extensive involvement of lung segments on CT scans.62,63
In a South Korean hospital-based study of 590 patients with newly diagnosed MAC-LD, 55% of patients had M. avium LD and 45% had M. intracellulare LD. Of those with M. avium LD, 45% received treatment within 3 years, compared with 65% of those with M. intracellulare LD. Risk factors for progression were FCD, positive AFB smear, and M. intracellulare LD.64 In another study from South Korea, 63% of 488 patients with MAC-LD received treatment within 3 years of diagnosis. In these patients, risk factors for progression included low BMI, systemic symptoms, FCD, positive AFB smear, and extensive disease. In total, 24% of patients did not receive treatment for ≥3 years following diagnosis, of whom 5% received treatment after 3 years of stable disease and 19% did not receive any treatment. Of those who received no treatment after 3 years, 52% had spontaneous negative culture conversion (representative of 10% of the full study population). Factors associated with spontaneous culture conversion were high BMI, negative AFB smear, and transient anti-TB medication for at least 1 month.49 A similar study in Taiwan retrospectively enrolled 450 patients with MAC-LD who had ≥2 positive sputum cultures. In total, 126 patients received microbiological follow-up, of which 60% had consistent positive culture and 40% had negative culture (representative of 11% of the full study population). Interestingly, only 12% of the study population received treatment, so the majority of negative conversions occurred without treatment. Factors associated with microbiological persistence included low BMI and positive AFB smear; conversely, factors associated with spontaneous negative culture conversion included high BMI and negative AFB smear.65 In a South Korean study of 1,021 patients with newly diagnosed non-cavitary NB MAC-LD or MABC-LD, 55% and 45% of patients were treated and untreated, respectively. Of those who were untreated, 65% had persistent positive culture, compared with 35% who had spontaneous negative culture conversion (representative of 15% of the full study population) (unpublished data).
Studies evaluating progression of MABC-LD, which was defined as initiation of therapy, showed that ≤47% of patients in South Korea14,66 and ≤55% of patients in Taiwan67,68 received treatment. The clinical outcomes of MAC and MABC-LD are very similar; as demonstrated in a hospital-based study in South Korea that followed 150 patients with NTM-LD (73% MAC, 21% MABC). Findings showed that there was no difference in 3-year progression rate (approximately 42%) between the two patient groups.69 Laboratory studies have suggested that there is an association between mycobacterial genotypes and disease progression,70-72 which could be useful for predicting the clinical course following diagnosis; however, genomic analysis is not available in routine clinical practice in most countries.
In summary, clinical studies report that approximately 40–60% of patients with MAC or MABC-LD are treated within 3 years of diagnosis and 40–60% are untreated. The most commonly reported risk factors associated with progression or treatment initiation are poor nutritional status (low BMI), increased disease severity (cavitary and extensive disease), and high bacterial burden (positive AFB smear). Between 35% and 50% of untreated patients (10–15% of all initially diagnosed patients) have spontaneous negative culture conversion, which is associated with a high BMI and low bacterial burden. Overall, the decision to treat NTM-LD should be based on the individual patient and the risks and benefits of treatment; those with cavitary disease should be treated following diagnosis, whereas those with non-cavitary disease should be assessed further to ascertain if treatment is necessary (general and nutritional conditions, symptoms, chest X-ray and CT scans, and bacterial burden).
To explore the burden of NTM-LD in Germany, Diel et al.6 carried out an analysis of 125 patients with NTM-LD, compared with 1,250 matched control patients. The incidence rate of NTM-LD was 2.6/100,000. The mean direct expenditure per patient with NTM-LD was four-fold that of matched controls (€39,559 versus €10,006), while hospitalisations were three times higher in patients with NTM-LD compared with matched controls and accounted for 63% of total costs. Attributable annual direct costs and indirect work- loss costs in patients with NTM-LD were €9,093 and €1,221 per patient, respectively. It was also found that 74% of patients with NTM-LD received antibiotics (29 different regimens were prescribed) and 12% were prescribed macrolide monotherapy. The mortality rate in patients with NTM-LD was higher than in matched controls, 22.4% and 6.0%, respectively, which was increased with concomitant COPD (41.5%).
Pathogenesis of Nontuberculous Mycobacterium Lung Disease
The pathogenesis of NTM-LD differs according to the pathogen, with species, subspecies, virulence/resistance mechanisms, and interactions with other pathogens all influencing factors. Environmental factors, such as temperature, evapotranspiration, pH, water chlorine content, biofilms, and soil mineral matter, also play a role, along with host factors, such as the presence of structural or genetic immunological diseases and certain phenotypes.
Microbiological Diagnosis of Nontuberculous Mycobacterium Lung Disease
Doctor Jakko van Ingen
Laboratory diagnosis of NTM-LD should involve mycobacterial culture on liquid and solid media at 30°C and 37°C, respectively, and partnership with a reference lab should allow culture with supplemented media, molecular identification of NTM to species level, and guideline-compliant drug susceptibility testing (DST). Molecular detection of resistance-conferring mutations and next-generation sequencing of NTM in clinical specimens should be on the research agenda. The clinical relevance of NTM isolates differs by species, so correct species identification is crucial to allow treatment to be evidence-based.
Although there are no validated commercial assays, a study comparing the correlation between polymerase chain reaction (PCR) and culture demonstrated that in smear-positive specimens, PCR detection of NTM had 100% sensitivity and 97% specificity, while in smear-negative specimens it had 50% sensitivity and 90% specificity.7 Identification of isolated NTM using molecular methods is important for rapid diagnosis and treatment decisions. Fibrocavitary disease (FCD) is easier to diagnose microbiologically, as sputum cultures are often positive and direct Ziehl–Neelsen staining of sputum often reveals acid-fast bacilli (AFB). Nodular bronchiectatic (NB)-LD is harder to diagnose as it has a lower bacterial burden and the sensitivity of direct staining, PCR, and culture is lower. A series of sputum samples or even a bronchoalveolar lavage sample may be required to find the causative NTM and meet the microbiological criteria for NTM-LD diagnosis.
Drug Susceptibility Testing
There is a myth that for NTM, results of in vitro DST do not correlate with outcomes of treatment in vivo.8-14 There is, however, sufficient evidence from clinical studies that susceptibility to macrolides and amikacin is correlated to outcomes of treatment. For rifampicin and ethambutol, which are prescribed for their synergy and not their individual effects, the activity against MAC in vitro is not important and should not be reported by laboratories.
Overall, it is important to have a multidisciplinary team to treat NTM-LD, including a pharmacist and a microbiology team who can identify to the species level and partner with reference laboratories. Quality control should be stringent and DST should be carried out according to existing guidelines.
Bronchiectasis as a Friend of Nontuberculous Mycobacterium and Vice Versa
Doctor Stefano Aliberti
Bronchiectasis is a disease with an increasing prevalence and incidence worldwide.15 Historically, it has been neglected, which has contributed to a lack of awareness and licenced treatments, with guidelines recommending reliance on real-life experience to manage the disease. Symptoms of bronchiectasis include chronic cough, haemoptysis, purulent sputum, wheeze, tiredness, and recurrent lower respiratory tract infections. However, it is a highly heterogeneous disease with variable aetiology, radiological findings, and pulmonary function. Chronic infection plays a crucial role in the vicious cycle of bronchiectasis pathophysiology; infection increases inflammation and leads to airway damage and development of bronchiectasis, which impairs lung defences and leaves the patient vulnerable to chronic infection.16
In 2016, the European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC)released a statement outlining research priorities in bronchiectasis, the third highest priority being the prevalence and characteristics of microbiological colonisation and chronic and acute infection (including NTM) in patients across Europe.17 The European Bronchiectasis Registry enrolled 9,123 patients from 27 countries and found that <5% of patients were infected with NTM.18
Nontuberculous Mycobacterium and Bronchiectasis: A Specific Clinical Phenotype
Among patients with bronchiectasis, there is a clear phenotype for those that have NTM: elderly (>60 years of age), female, with a low BMI, scoliosis, mitral valve prolapse, and no pre-existing LD. An interesting association exists between NTM-LD and cystic fibrosis transmembrane conductance regulator (CFTR) mutation. The clinical course is a prolonged cough with fatigue and weight loss, and radiology showing bronchiectasis with nodules particularly in the middle and upper lobes.
Prevalence of Nontuberculous Mycobacterium in Bronchiectasis
The prevalence of NTM in bronchiectasis varies between countries and study type (Table 1), and evaluation of the data is limited by low sample size, variable study periods, geographic locations, and the fact that most of the data are from case series, retrospective analyses, or databases.
Sputum Culture for Mycobacteria in Bronchiectasis
The Italian national audit of patients with bronchiectasis across 32 hospitals during 2014–2015 enrolled 1,361 patients (53% female; average age 70 years) and found that sputum culture during the stable period was performed in <30% of patients, and only 50% of cultures were for mycobacteria.25 In a prospective, observational, single-centre study from 2012–2015, 261 adults with bronchiectasis were identified (59% female; average age 69 years), of which 52% underwent bronchoscopy, 12% had NTM-LD (59% identified by fetal bovine serum and 41% from sputum), 4% had coinfection with Pseudomonas aeruginosa, and none were resistant to macrolides. Patients with NTM-LD were more likely to have cylindrical bronchiectasis (p=0.048), a ‘tree-in-bud’ pattern (p=0.011) on radiology, and a history of weight loss (p=0.045) compared with those infected with P. aeruginosa.23
Additionally, NTM-LD in bronchiectasis can be complicated by coinfection with other bacteria (such as P. aeruginosa), and fungi (such as Aspergillus).19,26 The presence of CFTR gene polymorphisms without cystic fibrosis (CF) may predispose to NTM infection by increasing the risk of bronchiectasis.27
American Thoracic Society Diagnostic Criteria
The American Thoracic Society (ATS) guidelines recommend that the following diagnostic criteria are met for diagnosis of NTM-LD: symptoms compatible with NTM-LD, radiology compatible with NTM-LD (e.g. cavities, nodules), ≥3 respiratory samples over ≥1 week, and ≥2 positive cultures with the same species (identified on both solid and liquid media plus molecular methods). For patients with bronchiectasis, the diagnosis of NTM-LD is very difficult as they already have the clinical and radiological criteria due to bronchiectasis.28
In conclusion, the diagnosis of NTM in patients with pre-existing bronchiectasis can be challenging. Cultures should be taken before macrolide therapy is initiated, and resistance should be monitored.
How to Assess and Manage the Co-Isolation of Nontuberculous Mycobacteria with Other Potential Respiratory Pathogens
Doctor Timothy Aksamit
The isolation of Mycobacterium tuberculosis and NTM is common, and the presence of multiple organisms is not rare. As a general rule, tuberculosis (TB) should be treated first and then NTM-LD reassessed. Notably, not all smear-positive disease is NTM (in the USA only 14% of smear-positive isolates were NTM).29 A recent report supported the recommendation to restrict investigations to sputum smear-positive patients;30 however, NTM should be considered when TB is being investigated. For example, in a cohort of patients with multiple drug-resistant TB in Cambodia, 409 patients were identified, of whom 46% were examined for culture. Two-thirds of the samples were culture-positive; 25% for NTM and 75% for M. tuberculosis.31
It is very common to isolate multiple NTM in one sample. For example, in a retrospective National Jewish Group study of 107 patients with NTM-LD, over half (55%) of patients had coexistent or previous MAC infection,13 whereas in another study of patients with CF, the initial isolate was almost always a single organism.32
A study of patients with MAC lung disease (MAC-LD) explored the significance of Mycobacterium abscessus subspecies abscessus (MAA) infection. In total, 29% of patients had MAA infection and the time from MAC-LD diagnosis to first MAA was between 9.9 and 11.3 months. Patients with MAA infections were more likely to have new or enlarging cavitary lesions on MAC therapy than those without coinfection (38% versus 0%, respectively).33 Treatment for MAC and MAA coinfection should include ethambutol, rifampin, a macrolide, amikacin, cefoxitin, and imipenem.
Aspergillus is a fungus that is more common in patients with NTM-LD than those without; the US Bronchiectasis Research Registry demonstrated that Aspergillus was present in 21% of patients with NTM-LD, versus 16% without.33 A cohort of patients with CF showed that 30% versus 8% of patients with and without NTM-LD, respectively, also had Aspergillus infection.32 Similarly, a retrospective study that compared 30 patients with NTM-LD and 61 patients with bronchiectasis without NTM-LD found that Aspergillus was present in 20% versus 0% of patients with and without NTM-LD, respectively.26 In the ATS Fungal Statement in 2011, voriconazole, itraconazole, posaconazole, amphotericin, and caspofungin were identified as treatments for Aspergillus infection;34 however, drug–drug interactions between NTM-LD and Aspergillus therapies limit the number of options. Although treatment with dose-adjusted antifungals and rifabutin has been carried out, along with therapeutic drug monitoring,35 it is not generally advised. Other options are to delay NTM treatment until Aspergillus infection is cured or to use alternative strategies that exclude rifamycin/rifabutin.
In conclusion, coinfection in NTM-LD is not uncommon, although the impact on natural history is yet to be established. Careful screening and regular monitoring for other pathogens is crucial for the care of patients with NTM-LD. Complex multidrug regimens that bear several drug–drug interactions are the only options currently available, and therapeutic drug monitoring should be utilised when treatment is prescribed.
Management of Nontuberculous Mycobacterium Treatment and New Strategies
Doctor David Griffith and Doctor Michael Loebinger
There are several factors that should be considered when making the decision whether to treat NTM-LD or not, such as whether NTM-LD is associated with cavities, the symptoms of the patient, pulmonary comorbidities, short and long-term prognosis, and the patient’s wishes. Additionally, at present there are a number of impediments to effective NTM-LD therapy, such as the long duration of therapy required, multiple drug cocktails, ongoing exposure to NTM from the environment, innate and acquired antibiotic resistance, poor clinical response to drugs, and patient factors (such as comorbid conditions).
Nontuberculous Mycobacterium Drug Resistance
Drug resistance can be either innate, i.e. natural drug resistance that is not readily associated with in vitro measures of resistance, such as minimal inhibitory concentrations (MIC) (for example, inducible macrolide resistance through the erm gene) or acquired, where isolates are selected with naturally occurring mutations that confer resistance to specific antibiotics. Some species of NTM, such as M. abscessus, MAC, and Mycobacterium simiae, show ‘cryptic’ resistance, whereby in vitro susceptibility results do not correlate with in vivo treatment response. However, some species, such as Mycobacterium kansasii (rmp), Mycobacterium marinum, Mycobacterium szulgai, and Mycobacterium fortuitum (erm), show good correlations between in vitro susceptibility and treatment response.
Treatment of Nontuberculous Mycobacterium
It is recommended to carry out initial in vitro susceptibility testing for NTM isolates to guide therapy, for example, to test the effectiveness of macrolides and amikacin against MAC. Gene mutations that confer resistance among NTM include the 23S rRNA gene and 16S rRNA gene in MAC, which confer resistance to macrolides and amikacin, respectively, the rpoβ gene in M. kansasii, which confers resistance to rifamycins, and the 23S rRNA gene in MAA/M. abscessus subspecies massiliense (MAM), which confers resistance to macrolides. Inadequate therapy in such cases results in acquired mutational resistance and can cause profound negative consequences for the treatment of NTM-LD.
In a study of 71 patients with M. massiliense LD, patients received oral macrolides along with 4 or 2 weeks initial intravenous (IV) amikacin and cefoxitin (or imipenem). Patients were treated for 24 months (4-week IV group) or for ≥12 months after negative sputum culture conversion (2-week IV group). Response rates after 12 months were 89% versus 100% for symptoms, 79% versus 91% for computed tomography (CT) scanning, and 100% versus 91% for negative sputum culture results in the 4 and 2-week IV groups, respectively. Acquired macrolide resistance developed in 2 patients in the 2-week IV group. Genotyping analysis for patients who failed to convert sputum culture to negative, or those with positive culture after successful treatment, revealed that most episodes were due to reinfection with different genotypes of M. massiliense.36 In an editorial by Griffith and Aksamit,37 the authors outlined that M. massiliense without an active erm gene is responsive to macrolide treatment, and that the primary mechanism of macrolide resistance is acquired mutational resistance. This is noteworthy as it is inevitable that some patients treated with macrolide monotherapy will develop resistance and become treatment-refractory.
The pharmacokinetic and pharmacodynamic indices of MAC therapy are often suboptimal, although currently there is no correlation with treatment outcome and no demonstrated correlation between circulating MAC drug levels and treatment outcome. There is also no correlation between MIC for rmp/emb/stm and response to therapy and no MIC cut-offs designating susceptible and resistant MAC strains for any antibiotics other than macrolides and amikacin. Additionally, inappropriate dependence on in vitro susceptibility results may have adverse consequences. Macrolides and amikacin treatment success for MAC correlates with in vitro MIC; with macrolides the susceptible MIC is ≤8 μg/mL and resistance MIC is ≥32 μg/mL, and with amikacin the susceptible MIC is ≤64 μg/mL and resistance MIC is >64 μg/mL.
The ATS guidelines on NTM recommend the following for NTM-LD for ≥12 months with sputum culture negativity on therapy:28
- NB disease: macrolide/ethambutol/ rifamycin; intermittent
- Cavitary disease: macrolide/EMB/ rifamycin ± injectable; daily
- Severe or previously treated disease: macrolide/EMB/rifamycin/injectable; daily
- Surgery for selected patients
Current treatment strategies for MAC-LD include a combination of rifampicin, ethambutol, azithromycin, and clarithromycin with the addition of IV amikacin or ≤3 months nebulised amikacin for severe cases. Antibiotic treatment should continue for ≥12 months after culture conversion (unpublished data). Treatment of M. abscessus LD should involve an initial phase of ≥1 month of IV amikacin, IV tigecycline, and, where tolerated, IV imipenem plus oral macrolide followed by a continuation phase of nebulised amikacin, oral macrolide, and 1–3 oral antibiotics guided by drug susceptibility and patient tolerance. Despite use of these regimens, mortality remains high, with a 5-year all-cause mortality reported between 23–70% in a variety of retrospective cohort studies of different mycobacterial species.38-41 In addition to the high mortality rates, significant numbers of patients remain refractory with continued isolation of mycobacteria. The highest culture conversion success rates are generally with M. kansasii, and the worst outcomes for Mycobacterium xenopi or M. abscessus. A recent meta-analysis of 16 studies and 1,462 patients suggested a sustained conversion rate of 60% in MAC-LD.42,43
A retrospective single-centre study aimed to validate the appropriateness of current treatment for MAC-LD. In total, 180 patients with NB-LD completed >12 months of macrolide/azalide multidrug therapy. Sputum conversion to culture-negativity occurred in 86% of patients, with no differences between clarithromycin or azithromycin regimens. Treatment regimen modification occurred more frequently with daily (80%) versus intermittent (1%) therapy. Microbiological recurrences during therapy occurred in 14% of patients: 73% with reinfection MAC isolates and 27% with true relapse isolates. Overall treatment success was achieved in 84% of patients. Following completion of therapy, microbiological recurrences occurred in 48%: 75% reinfection isolates and 25% true relapse isolates.44 A similar study in South Korea of 217 patients with NB MAC-LD found that rates of symptomatic improvement, radiographic improvement, and sputum culture conversion were not different between daily therapy and intermittent therapy (75% versus 82%, 68% versus 73%, and 76% versus 67%, respectively). Modification of the initial antibiotic regimen occurred more frequently in the daily therapy group than in the intermittent therapy group (46% versus 21%).45
Overall, current guidelines for macrolide/azalide-based regimens for NB MAC-LD show favourable microbiological outcomes and do not promote macrolide resistance. Daily and intermittent regimens are equally effective, although intermittent regimens have fewer side effects. Microbiological recurrences are common and mostly due to unique MAC genotypes. However, there is a lack of adherence to guidelines in the USA, Europe, and Japan.46,47
Cavitary Mycobacterium Avium Complex Nontuberculous Mycobacterium Lung Disease
Cavitary MAC NTM-LD is a smoking COPD-related disease that is likely associated with long-term respiratory impairment and requires aggressive therapy. Appropriate therapy includes parenteral agents, surgery, smoking cessation, and avoidance of macrolide resistance. Patients should be advised that smoking during treatment can inhibit a favourable treatment response.
A National Institutes of Health (NIH) study of mortality among patients with NTM-LD demonstrated that FCD and pulmonary hypertension (PH) were significant risk factors for death in NTM-LD. Median survival for patients with FCD was 9.0 years versus 13.1 years with no FCD (p=0.006), and 6.8 years for patients with PH versus >18 years for those without PH (p=0.048).48
Surgery for Nontuberculous Mycobacterium Lung Disease
Surgery is indicated for NTM-LD if medication is unresponsive (due to resistance or large cavities) or if the patient has uncontrolled symptoms, haemoptysis, or highly damaged lung tissue.
The Nontuberculous Mycobacterium Lung Disease Drug Pipeline
New drugs for NTM-LD should be tested using sputum conversion, duration of therapy, and symptomatic improvement as endpoints. Currently, liposomal amikacin inhalation (LAI) is under investigation for the treatment of NTM-LD and the U.S. Food and Drug Administration (FDA)-approved drugs (bedaquiline, linezolid, tedizolid, clofazimine) are being studied for potential use.
There is some evidence that a proportion of patients with stable NTM-LD do not require treatment and culture-convert spontaneously. In an observational study by Hwang et al.,49 93 patients (out of 420) with MAC-LD were untreated and followed up for a median of 5.6 years. Of these patients, 51.6% showed spontaneous sputum conversion.
Nebulised Amikacin
A study of nebulised amikacin in 20 patients with MAC or M. abscessus suggested some response to therapy, with 5 patients demonstrating negative cultures after treatment.50
Liposomal Amikacin Inhalation
In a Phase II study, the efficacy and safety of LAI 590 mg once-daily versus placebo was assessed for 84 days with an additional open-label treatment period of 84 days. The primary endpoint was reduction in NTM growth by semi-quantitative sputum cultures. The primary endpoint was not achieved, although a greater proportion of patients in the LAI group demonstrated ≥1 negative sputum culture (32% versus 9%; p=0.006) and improvement in 6-minute-walk test at Day 84. Most adverse events were respiratory (hoarseness and bronchospasm) and led to drug discontinuation in some patients. Of those who achieved culture conversion after 3–6 months of LAI, 82.4% had negative sputum culture at 12 months after LAI discontinuation.11
The Phase III CONVERT study of LAI therapy enrolled 336 patients with NTM-LD caused by MAC who were treatment refractory for ≥6 months of guideline-based therapy (GBT). Top line results have been released that show the primary endpoint, culture conversion, was met (29% LAI + GBT versus 9% GBT-placebo; p<0.0001).51
Bedaquiline
Bedaquiline is a drug used to treat TB and has been investigated for potential use in NTM-LD. A study of untreated MAC isolates MIC90 with bedaquiline 0.004–0.008 μg/mL using Clinical and Laboratory Standards Institute methods demonstrated no difference between M. avium and Mycobacterium intracellulare.52 Preliminary results of an open case series of off-label bedaquiline use in 10 patients with MAC or M. abscessus LD showed some symptomatic benefit; however, microbiological and radiological outcomes were mixed.53
Linezolid and Tedizolid
Linezolid is indicated in the British Thoracic Society (BTS) guidelines for possible use against M. abscessus disease, although clinical evidence of efficacy is lacking. One study assessed the tolerability of linezolid for NTM-LD; cytopenia, peripheral neuropathy, and optic neuropathy were identified as significant adverse events.54
In a further study, the potency of tedizolid was compared with linezolid using in vitro testing of 170 NTM isolates, including resistant strains. Tedizolid demonstrated a greater in vitro potency compared with linezolid (Table 2), which indicated its use as a potential treatment for NTM infections.55
Clofazimine-containing regimens have been shown to be effective when compared with rifampin-containing regimens for the treatment of MAC-LD in a study of 107 patients and may be a suitable alternative.56
Other Strategies
In addition to new drugs, different treatment paradigms may be needed to improve treatment success. Presently, treatment is advised for 1 year following culture conversion. Despite apparent treatment success, high relapse and reinfection rates ≤48% have been reported.44 It is possible that longer treatment periods may be needed for patients with higher relapse rates (such as those with cavitary disease) or prophylactic treatment post eradication may reduce the risk of reinfection. NTM are ubiquitous in the environment, so preventing exposure is challenging; however, certain cleaning regimens may be more effective at environmental eradication of NTM.
An increase in precision medicine and targeting specific treatments to certain individuals is likely to occur in the future. For example, patients with specific predispositions or susceptibilities to NTM-LD may benefit from a treatment approach that addresses the host in addition to the microbe, such as with the use of immunomodulators. Additionally, as we learn more about the microbes with technologies such as sequencing, treatments directly affecting virulence targets, biofilms, and the microbiome are likely to develop.
The Natural History of Untreated Nontuberculous Mycobacterium Lung Disease: A Global Perspective
Doctor Won-Jung Koh
The natural history of untreated pulmonary TB was studied in 126 AFB smear-positive patients in South India from 1961–1968 when no treatment was available. At the end of the study, 49% of patients had died, 18% had chronic disease, and 33% were cured without treatment.57 This study was not powered to detect NTM; however, more recent studies have provided better understanding of the natural history of NTM-LD.
MAC (M. avium and M. intracellulare) and M. abscessus complex (MABC) (M. abscessus and M. massiliense) are the most common causes of NTM-LD (approximately 90% of cases); therefore, natural history studies have focussed on these four pathogens. Fibrocavitary NTM-LD is typically caused by M. intracellulare and M. massiliense and patients are generally middle-aged/elderly males with underlying pulmonary disorders such as COPD and TB. Chest X-ray and CT scans show thin-walled apical cavity in the upper lobes of the lung, which, if left untreated, can progress in 1–2 years to cause extensive lung damage and death. NB NTM-LD is typically caused by M. intracellulare and patients are generally middle-aged/elderly females without underlying pulmonary disorders, who do not smoke. A high-resolution CT scan shows multiple small nodules (<5 mm) and bronchiectasis in both lungs, and the course of disease is indolent with slow progression.
Mycobacterium Avium Complex Lung Disease Mortality
In a retrospective study of 634 Japanese patients with MAC-LD, 76%, 21%, and 3% had NB-LD, fibrocavitary LD, or other forms of NTM-LD, respectively. Kaplan-Meier curves of all-cause mortality according to radiographic features showed that the survival rate of patients with FCD was significantly lower than those with NB-LD or other forms of NTM-LD (p<0.001).40 In a similar study of 782 patients with NB MAC-LD, 85% had non-cavitary disease and 15% of patients had cavitary disease. Ten-year survival rates were significantly lower for patients with cavitary disease (25%) compared with those without cavitary disease (0.8%); (p<0.001).58 Taken together, these results demonstrate that cavitation is an important prognostic factor in both FCD and NB-LD.
Nontuberculous Mycobacterium Lung Disease Treatment Decision
The ATS guidelines state that a diagnosis of NTM-LD does not necessarily lead to the initiation of treatment; they recommend that the decision to initiate treatment for patients with NTM-LD should be based on risk-to-benefit analysis that takes into account patient symptoms, radiographic findings (such as severity and progression), and microbiological results versus the adverse effects of multiple potentially toxic drugs.28 In clinical practice, the proportion of patients who receive treatment for NTM-LD varies according to the country: population-based studies in the USA, the Netherlands, and Germany found that 19%, 57%, and 75% of patients received treatment, respectively.6,59,60
Mycobacterium Avium Complex Lung Disease and Mycobacterium Abscessus Complex Lung Disease Progression
Hospital-based studies evaluating progression of MAC-LD (defined as initiation of therapy) showed that 43% of 392 patients in the USA were started on treatment,61 53% of 72 patients with NB-LD in Japan showed progressive disease on chest X-ray at 10 years follow-up,62 and 51% of 67 patients with NB-LD in South Korea had progressive disease at 5-year follow-up.63 In these patients, risk factors for progression included low BMI, cavitation, and extensive involvement of lung segments on CT scans.62,63
In a South Korean hospital-based study of 590 patients with newly diagnosed MAC-LD, 55% of patients had M. avium LD and 45% had M. intracellulare LD. Of those with M. avium LD, 45% received treatment within 3 years, compared with 65% of those with M. intracellulare LD. Risk factors for progression were FCD, positive AFB smear, and M. intracellulare LD.64 In another study from South Korea, 63% of 488 patients with MAC-LD received treatment within 3 years of diagnosis. In these patients, risk factors for progression included low BMI, systemic symptoms, FCD, positive AFB smear, and extensive disease. In total, 24% of patients did not receive treatment for ≥3 years following diagnosis, of whom 5% received treatment after 3 years of stable disease and 19% did not receive any treatment. Of those who received no treatment after 3 years, 52% had spontaneous negative culture conversion (representative of 10% of the full study population). Factors associated with spontaneous culture conversion were high BMI, negative AFB smear, and transient anti-TB medication for at least 1 month.49 A similar study in Taiwan retrospectively enrolled 450 patients with MAC-LD who had ≥2 positive sputum cultures. In total, 126 patients received microbiological follow-up, of which 60% had consistent positive culture and 40% had negative culture (representative of 11% of the full study population). Interestingly, only 12% of the study population received treatment, so the majority of negative conversions occurred without treatment. Factors associated with microbiological persistence included low BMI and positive AFB smear; conversely, factors associated with spontaneous negative culture conversion included high BMI and negative AFB smear.65 In a South Korean study of 1,021 patients with newly diagnosed non-cavitary NB MAC-LD or MABC-LD, 55% and 45% of patients were treated and untreated, respectively. Of those who were untreated, 65% had persistent positive culture, compared with 35% who had spontaneous negative culture conversion (representative of 15% of the full study population) (unpublished data).
Studies evaluating progression of MABC-LD, which was defined as initiation of therapy, showed that ≤47% of patients in South Korea14,66 and ≤55% of patients in Taiwan67,68 received treatment. The clinical outcomes of MAC and MABC-LD are very similar; as demonstrated in a hospital-based study in South Korea that followed 150 patients with NTM-LD (73% MAC, 21% MABC). Findings showed that there was no difference in 3-year progression rate (approximately 42%) between the two patient groups.69 Laboratory studies have suggested that there is an association between mycobacterial genotypes and disease progression,70-72 which could be useful for predicting the clinical course following diagnosis; however, genomic analysis is not available in routine clinical practice in most countries.
In summary, clinical studies report that approximately 40–60% of patients with MAC or MABC-LD are treated within 3 years of diagnosis and 40–60% are untreated. The most commonly reported risk factors associated with progression or treatment initiation are poor nutritional status (low BMI), increased disease severity (cavitary and extensive disease), and high bacterial burden (positive AFB smear). Between 35% and 50% of untreated patients (10–15% of all initially diagnosed patients) have spontaneous negative culture conversion, which is associated with a high BMI and low bacterial burden. Overall, the decision to treat NTM-LD should be based on the individual patient and the risks and benefits of treatment; those with cavitary disease should be treated following diagnosis, whereas those with non-cavitary disease should be assessed further to ascertain if treatment is necessary (general and nutritional conditions, symptoms, chest X-ray and CT scans, and bacterial burden).








