Chairperson: David Price1-3
Speakers: David Price,1-3 Claus Vogelmeier,4 Kenneth Chapman5,6
1. University of Aberdeen, Aberdeen, UK
2. Observational & Pragmatic Research Institute, Southbank, Singapore
3. Optimum Patient Care, Oakington, UK
4. Department of Pulmonology, Philipps University of Marburg, Marburg, Germany
5. University of Toronto, Toronto, Canada
6. Asthma and Airway Centre, University Health Network, Toronto, Canada
Disclosure: Prof Price has received grants, research support, honoraria/consultation fees, and/or other payments from Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Circassia, GlaxoSmithKline, Kyorin, Mylan, Merck & Co., Mundipharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Respiratory Effectiveness Group, Sanofi Genzyme, Teva Pharmaceuticals, Theravance, and the UK National Health Service (NHS); has participated in company sponsored bureaus for Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, and Teva Pharmaceuticals; and is a stock shareholder in AKL Research and Development Ltd. Prof Vogelmeier has presented at symposia and/or served on scientific advisory boards sponsored by AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Grifols, Novartis, Menarini, and MedUpdate. Philipps University of Marburg has received unrestricted grants from GlaxoSmithKline, Grifols, and AstraZeneca. Prof Chapman has participated in consultations for, delivered lectures, and/or received research grants/contracts from Amgen, AstraZeneca, Boehringer Ingelheim, CSL Behring, Genentech, GlaxoSmithKline, Gossamer, Grifols, InhibRx, Kamada, Merck Frosst, Novartis, Regeneron, Roche, Sanofi, and Takeda.
Acknowledgements: Writing assistance was provided by Helen Boreham, HB Medical UK Ltd, Kent, UK.
Support: The publication of this article was funded by Novartis. The views and opinions expressed are those of the presenters. Content was reviewed by Novartis for
medical accuracy.
Citation: EMJ Respir. 2020;8[Suppl 1]:2-11.
Meeting Summary
Despite clinical advances reducing the number of asthma-related deaths, significant unmet needs remain. Treatment mismatch, poor adherence, and treatment misuse all contribute to the problem of uncontrolled asthma. During this symposium, leading respiratory disease experts reconsidered the potential of inhaled therapies in the management of asthma, focussing on the treatment opportunity afforded by a new once-daily, fixed-dose combination of indacaterol acetate (a long-acting β2-agonist [LABA]), glycopyrronium bromide (a long-acting muscarinic antagonist [LAMA]), and mometasone furoate (an inhaled corticosteroid [ICS]) delivered via the Breezhaler® device (Novartis, Basel, Switzerland). The scope for new devices and digital solutions to further improve treatment use and adherence in the asthma arena was also discussed.
Introduction to the PLATINUM Programme
Professor David Price
Globally, an estimated 300 million people have asthma, which ranks as one of the top 30 causes of disability-adjusted life years and is among the top 10 causes in younger patients aged 5–14 years.1-3 Many of those with severe disease live in fear of potential exacerbations that remain highly prevalent despite current best therapies.4 Exacerbations are important, not just because of their associated mortality burden, but because of the increased risk of side effects from systemic corticosteroids (SCS) used in treatment. A recent long-term observational study revealed a strong, dose-dependent link between lifetime SCS use and the development of comorbidities such as osteoporosis and Type 2 diabetes mellitus.5
Evidence has indicated that both healthcare professionals (HCP) and patients have overestimated the level of disease control currently achieved in asthma.6,7 In reality, more than one-third of patients with asthma remain uncontrolled despite treatment because of issues with adherence or treatment misuse, principally poor inhaler technique.8 Nonadherence rates may reach as high as 70% among patients with asthma and approximately 50% of patients with asthma are still not managing to use their inhaler correctly despite training.9,10 This problem is compounded by the fact that HCP lack objective data on patients’ self-management and adherence, which would support more effective decision-making in everyday clinical practice.9,11
Two new once-daily, fixed-dose inhaled combinations have recently been added to the treatment armoury for asthma: indacaterol acetate and mometasone furoate (IND/MF); and indacaterol acetate, glycopyrronium bromide, and mometasone furoate (IND/GLY/MF). IND/MF (LABA/ICS) is available in three ICS dose strengths: low, medium, and high, while IND/GLY/MF (LABA/LAMA/ICS) is available at medium and high ICS doses. Both are delivered through the Breezhaler device.
These new therapies are underpinned by an extensive Phase III clinical trial programme, PLATINUM, involving over 7,500 patients. Key studies within the PLATINUM programme include the PALLADIUM, IRIDIUM, and ARGON studies (Table 1).12-14
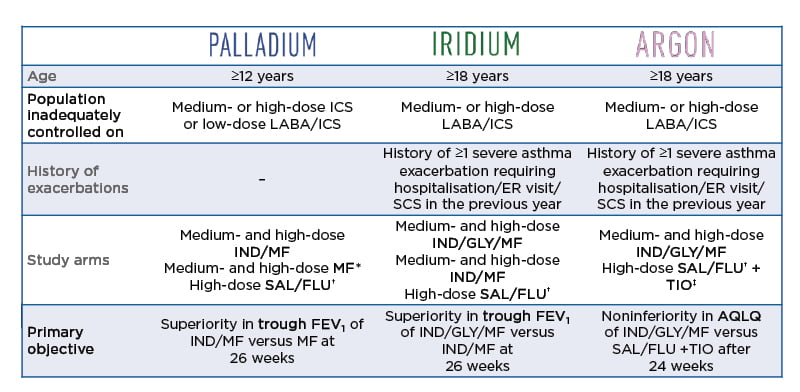
Table 1: The PLATINUM indacaterol acetate, glycopyrronium bromide, and mometasone furoate and indacaterol acetate and mometasone furoate asthma programme.
Note: exacerbations requiring SCS, hospitalisation, or emergency room visit <6 weeks prior to screening were excluded.
*Medium-dose MF 400 µg once daily; high-dose MF 400 µg twice daily.
†High-dose SAL/FLU 50/500 µg twice daily.
‡TIO 5 µg.
ACQ: Asthma Control Questionnaire; AQLQ: Asthma Quality of Life Questionnaire; ER: emergency room; FEV1: forced expiratory volume in 1 second; IND/GLY/MF high dose: indacaterol acetate/glycopyrronium bromide/mometasone furoate 150/50/160 µg once daily; IND/GLY/MF medium dose: indacaterol acetate/glycopyrronium bromide/mometasone furoate 150/50/80 µg once daily; IND/MF high dose: indacaterol acetate/mometasone furoate
150/320 µg once daily; IND/MF medium dose: indacaterol acetate/mometasone furoate 150/160 µg once daily; MF high/medium: mometasone furoate 800/400 µg once daily; SAL/FLU: salmeterol/fluticasone propionate 50/500 µg twice daily; SCS: systemic corticosteroids; TIO: tiotropium 5 µg once daily.
Adapted from van Zyl-Smit et al.12, Kerstjens et al.,13 and Gessner et al.14
A New LABA/ICS: A New Treatment Opportunity
Professor Claus Vogelmeier
Prof Vogelmeier shared data from the PALLADIUM trial, a 52-week, randomised, double-blind, Phase III study, which compared IND/MF to MF and salmeterol/fluticasone propionate (SAL/FLU). The study population included adult/adolescent patients with asthma aged 12–75 years who were inadequately controlled on medium- or high-dose ICS or low-dose LABA/ICS, but had few exacerbations.12 Patients’ mean Asthma Control Questionnaire (ACQ-7) score at baseline was 2.3. The study had five arms: IND/MF (medium and high dose once daily via Breezhaler device), MF (medium [once daily] and high dose [twice daily]) via Twisthaler® [Novartis, Basel, Switzerland]), and high-dose SAL/FLU (twice daily via Diskus® [GlaxoSmithKline, Middlesex, UK]). Rescue medication was available throughout the study.
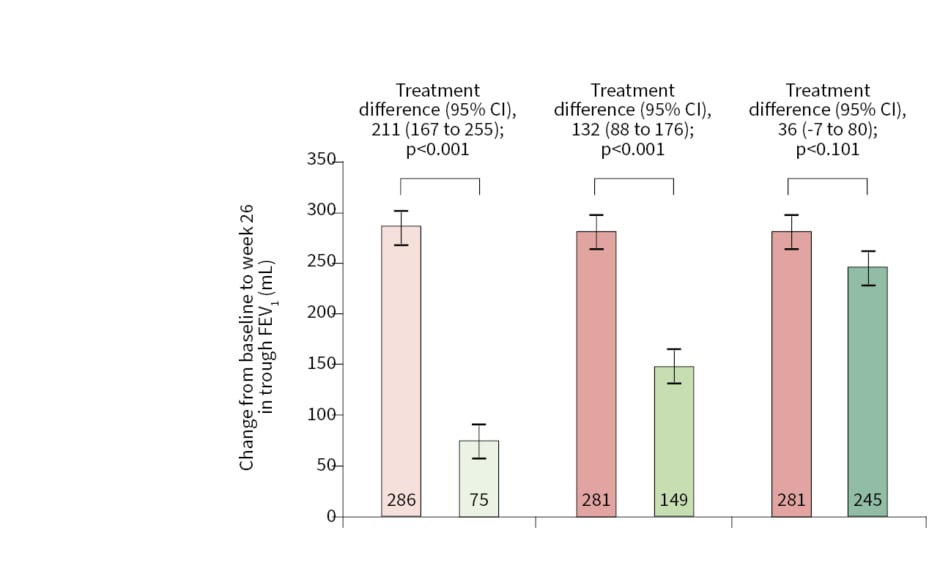
PALLADIUM met its primary endpoint (Figure 1),12,15 with both doses of IND/MF significantly increasing trough forced expiratory volume in 1 second (FEV1) versus corresponding doses of MF at Week 26. Significant improvements in FEV1 of 211 mL and 132 mL were demonstrated by medium and high doses of IND/MF versus MF, respectively. High-dose IND/MF also significantly improved trough FEV1 by 48 mL compared to SAL/FLU at 52 weeks.12,15
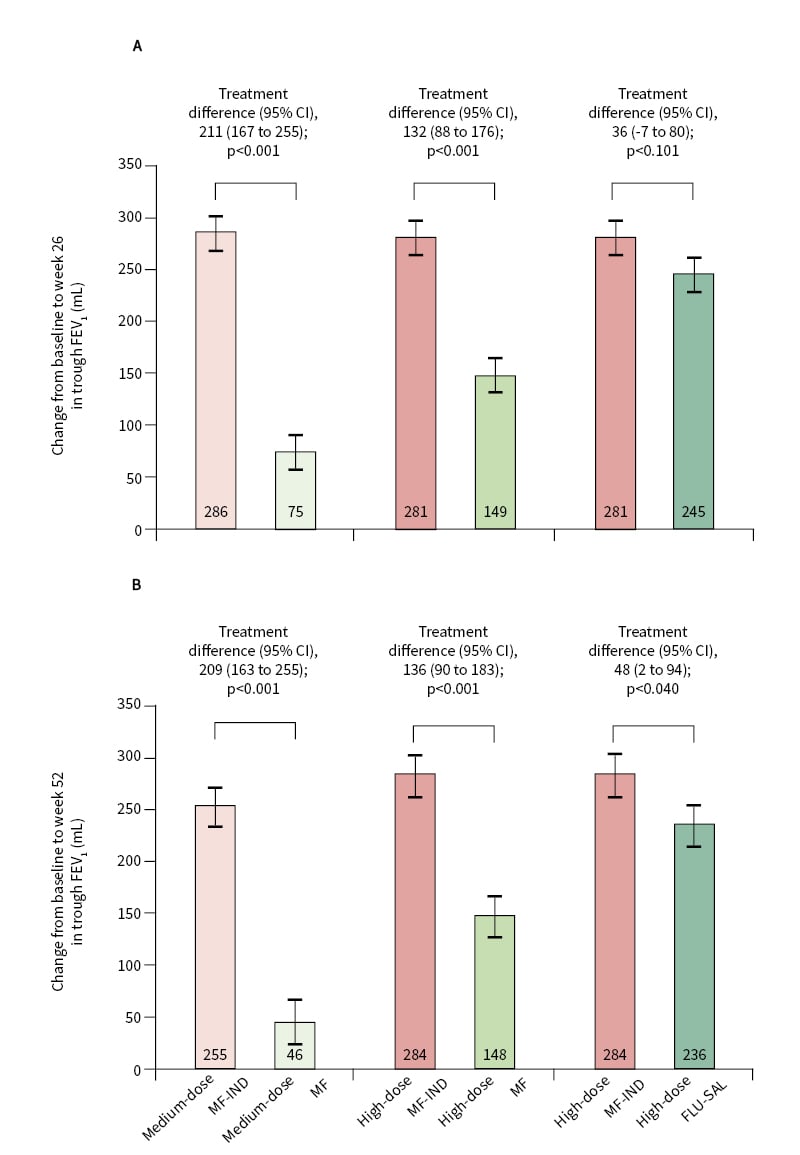
Figure 1: PALLADIUM primary endpoint (image from source article and not presentation described).
Change from baseline in trough FEV1 at week 26 and week 52 in the full analysis set.
Change in trough FEV1 at week 26 (A) and week 52 (B) after treatment with MF-IND versus MF and FLU-SAL. Participants received high-dose MF-IND (320 µg, 150 µg) once daily; or medium-dose MF-IND (160 μg, 150 µg) once daily; or high-dose MF (800 µg) 400 µg twice daily; or medium-dose MF (400 µg) once daily; or high-dose FLU-SAL (500 µg, 50 µg) twice daily.
CI: confidence interval; FLU-SAL: fluticasone propionate/salmeterol xinafoate; MF: mometasone furoate; MF-IND: mometasone furoate/indacaterol acetate.
Reprinted from The Lancet Respiratory Medicine, epub ahead of print, van Zyl-Smit et al., Once-daily mometasone plus indacaterol versus mometasone or twice-daily fluticasone plus salmeterol in patients with inadequately controlled asthma (PALLADIUM): a randomised, double-blind, triple-dummy, controlled Phase 3 study, Copyright (2020), with permission from Elsevier.12
In terms of symptom control, a key secondary endpoint, both doses of IND/MF significantly improved ACQ-7 score versus MF at Week 26, with 0.248 and 0.171 point increases for medium and high doses, respectively.12,15 Improvements in ACQ-7 score were comparable between high-dose IND/MF and high-dose SAL/FLU at Week 26.12,15 Both doses of IND/MF also significantly boosted the proportion of rescue medication-free days (by 8.6─9.6%) over 52 weeks compared to MF.12,15 A significant 4.3% increase in rescue medication-free days was seen for the high-dose IND/MF treatment group versus SAL/FLU.12,15
As the PALLADIUM population was not exacerbation-enriched, a prespecified pooled analysis of both PALLADIUM and IRIDIUM trial data was performed to examine this key
outcome.16 High-dose IND/MF was shown to significantly improve exacerbations versus high-dose SAL/FLU, achieving reductions in the annualised rate of severe, moderate/severe, and all exacerbations of 26%, 22%, and 19%, respectively.16
Safety findings from the PALLADIUM study indicated that IND/MF was well tolerated, with comparable toxicity profiles seen across the study arms.12 The overall incidence of adverse events (AE) and AE known to be specifically associated with ICS and/or LABA, including the clinically relevant side effect of pharyngitis, was generally balanced across treatment groups.12
Reconsidering the Potential of Inhaled Therapies with a New Once-Daily, Fixed LAMA/LABA/ICS Combination: Part One
Professor Claus Vogelmeier
Another key study from the Phase III PLATINUM programme was IRIDIUM, a 52-week, randomised, double-blind trial assessing IND/GLY/MF against IND/MF or high-dose SAL/FLU in patients with asthma.13 IRIDIUM had five study arms: IND/GLY/MF (medium and high dose once daily via Breezhaler device), IND/MF (medium and high dose once daily via Breezhaler device), and SAL/FLU (high dose twice daily via Diskus). Unlike the PALLADIUM population, IRIDIUM enrolled adults (≥18 years old) with asthma that was poorly controlled on medium- or high-dose LABA/ICS, with a history of at least one severe exacerbation requiring SCS in the prior year. Mean ACQ-7 score at baseline
was 2.5. Rescue medication was available throughout the study.
Both doses of IND/GLY/MF achieved significant improvements in trough FEV1 versus IND/MF at Week 26, meeting the study’s primary endpoint (Figure 2).13,17 IND/GLY/MF improved FEV1 by 76 mL and 65 mL at medium and high doses, respectively, versus comparable doses of IND/MF at Week 26. These significant improvements in lung function were maintained to Week 52, confirming no signs of tachyphylaxis in the first year of treatment.13,17
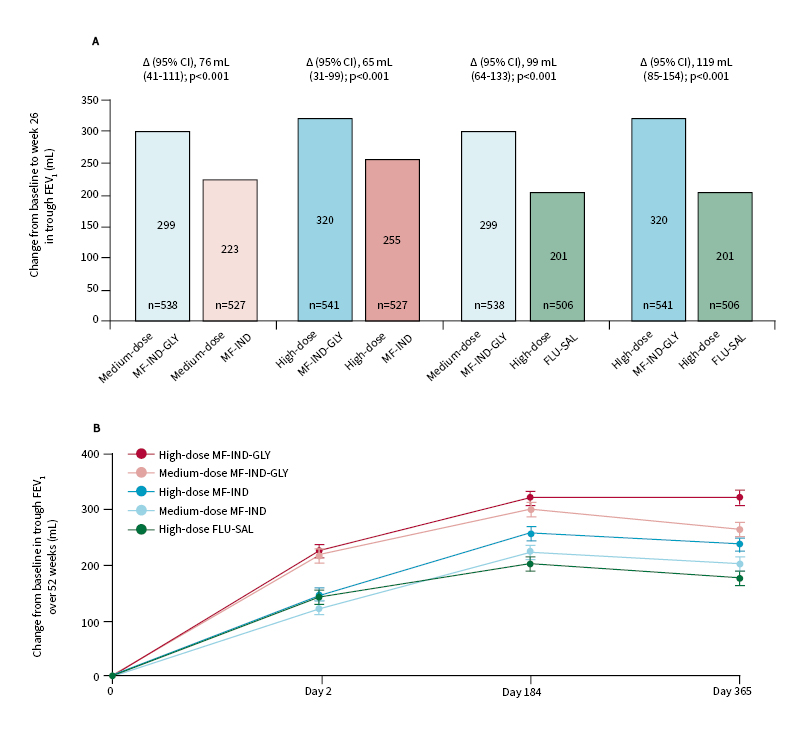
Figure 2: IRIDIUM primary endpoint (image from source article and not presentation described).
Change from baseline in trough FEV1 at week 26 and week 52 in the full analysis set.
Change in trough FEV1 with MF-IND-GLY versus MF-IND and FLU-SAL at week 26 (A) and over week 52 (B). Data are presented as least squares mean (SE); error bars represent SE values. Patients received medium-dose MF-IND-GLY (80 µg, 150 µg, 50 µg) once daily; or high-dose MF-IND-GLY (160 µg, 150 µg, 50 µg) once daily; or medium-dose MF-IND (160 µg, 150 µg) once daily; or high-dose MF-IND (320 µg, 150 µg) once daily; or high-dose FLU-SAL (500 µg, 50 µg) twice daily. Δ=treatment difference. CI: confidence interval; FEV1: forced expiratory volume in 1 sec; FLU-SAL: fluticasone/salmeterol; MF-IND: mometasone/indacaterol; MF-IND-GLY: mometasone/indacaterol/glycopyrronium; SE: standard error.
Reprinted from The Lancet Respiratory Medicine, epub ahead of print, Kerstjens et al., Once-daily, single-inhaler mometasone–indacaterol–glycopyrronium versus mometasone–indacaterol or twice-daily fluticasone–salmeterol in patients with inadequately controlled asthma (IRIDIUM): a randomised, double-blind, controlled Phase 3 study, Copyright (2020), with permission from Elsevier.13
Important secondary endpoints evaluated in the IRIDIUM trial included exacerbation rate and symptom control. High-dose IND/GLY/MF significantly reduced the rate of severe and total exacerbations compared to high-dose IND/MF, cutting the annualised rate by >20%.13,17 This positive effect on exacerbations was confirmed in post hoc analysis of the IRIDIUM trial, where high-dose IND/GLY/MF was shown to significantly lower exacerbations after 52 weeks, with a 21% and 31% cut in moderate/severe and severe exacerbations, respectively, suggesting a dose-effect relationship (Novartis. Data on file). Although significant improvements in ACQ-7 score, a key secondary endpoint, were not observed for IND/GLY/MF versus corresponding doses of IND/MF at Week 26, all treatment arms demonstrated similarly high and clinically relevant improvement in change
from baseline.13
Prof Vogelmeier concluded that, overall, findings from the PALLADIUM and IRIDIUM trials demonstrated the ability of the new combinations of IND/MF and IND/GLY/MF to significantly increase lung function, improve symptom control, and reduce exacerbations.
Reconsidering the Potential of Inhaled Therapies with a New Once-Daily, Fixed LAMA/LABA/ICS Combination: Part Two
Professor Ken Chapman
IRIDIUM: IND/GLY/MF Versus SAL/FLU
In the second presentation on the IRIDIUM trial, data directly comparing IND/GLY/MF to
SAL/FLU were reviewed by Prof Chapman, addressing the important question of whether IND/GLY/MF can provide meaningful benefit to patients with uncontrolled asthma already receiving high-dose ICS/LABA.
Both doses of IND/GLY/MF improved trough FEV1 compared to SAL/FLU.13,17 At Week 26, there was a significant improvement in FEV1 of 99 mL for medium-dose IND/GLY/MF and 119 mL for high-dose IND/GLY/MF versus high-dose SAL/FLU. Corresponding FEV1 improvements with medium- and high-dose IND/GLY/MF at Week 52 were 87 mL and 145 mL, respectively, representing a clinically important and statistically significant difference.13,17
Significant reductions in exacerbations were also seen with high-dose IND/GLY/MF versus high-dose SAL/FLU across the board, with reductions in the annualised rates of severe, moderate/severe, and all exacerbations of 42%, 36%, and 40%, respectively.13,17 Although no significant impact on the rate of severe exacerbations was seen with medium-dose IND/GLY/MF versus high-dose SAL/FLU, the sum of all exacerbations was significantly reduced by 30%.13,17
IND/GLY/MF also met the secondary endpoint of symptom reduction, with both doses showing a small but statistically significant improvement in ACQ-7 score over SAL/FLU at Week 26.13 Similarly, a higher proportion of patients experienced a clinically important improvement in their symptom scores (an improvement in ACQ-7 of >0.5 points) with the three-drug combination. Treatment with high-dose IND/GLY/MF significantly boosted the total number of ACQ-7 responders by 23.7%, 11.2%, and 8.2% at Weeks 4, 12, and 52, respectively, versus high-dose SAL/FLU.13,17
With regard to its safety profile, IND/GLY/MF proved well tolerated, with the overall incidence of AE and serious AE comparable across treatment groups in the IRIDIUM trial.13
In summary, IRIDIUM findings showed that high-dose IND/GLY/MF achieved greater improvement in lung function, greater reduction in exacerbations, and greater improvement in patient symptoms compared to high-dose SAL/FLU, with a comparable safety profile. Prof Chapman noted that similar results were seen for medium-dose IND/GLY/MF, albeit with a nonsignificant trend towards greater reduction in severe exacerbations.
The ARGON Trial and Real-World Evidence
Prof Chapman acknowledged that, despite the compelling data from the IRIDIUM trial, clinicians may question whether it is feasible to simply use a LAMA in free combination with an ICS-LABA instead of the closed, fixed-dose combination of IND/GLY/MF. This important question was addressed in the Phase III ARGON study: a 24-week, randomised, partially-blinded, noninferiority study of IND/GLY/MF (medium and high dose once daily via Breezhaler device) versus the free combination of SAL/FLU (high dose twice daily via Diskus), plus external tiotropium ([TIO] in its soft mist inhaler format once daily). The ARGON study considered patients that were poorly controlled and experiencing exacerbations;14 specifically, patients were aged ≥18 years and had inadequately controlled asthma on medium- or high-dose LABA/ICS, with a history of ≥1 severe asthma exacerbation requiring medical care from a physician, emergency room visit, or hospitalisation, and requiring SCS for ≥3 days in the previous year. Patients’ mean ACQ-7 score at baseline was 2.6.
The primary endpoint of ARGON consisted of a noninferiority comparison that showed both doses of IND/GLY/MF to be noninferior to high-dose SAL/FLU plus TIO, achieving comparable improvement in asthma-related quality of life scores after 24 weeks.14 For the secondary endpoint of trough FEV1, high-dose IND/GLY/MF produced a significant improvement of 96 mL over high-dose SAL/FLU plus TIO at Week 24.14
Looking at the clinically-relevant impact on exacerbations and symptoms, high-dose IND/GLY/MF significantly reduced the rate of moderate exacerbations by 43% compared to high-dose SAL/FLU plus TIO over 24 weeks, which was an exploratory endpoint in the ARGON trial.14 Reductions in the rate of total and moderate or severe exacerbations were comparable between both doses of IND/GLY/MF and high-dose SAL/FLU plus TIO.14 Patients treated with the novel three-drug combination also benefited from an improvement in ACQ-7 symptom scores, which was statistically significant for high dose IND/GLY/MF versus high-dose SAL/FLU plus TIO.14 Again, safety profiles were comparable across the different study arms.
The real-world data for open triple therapy further strengthened the rationale for choosing a fixed-dose combination therapy taken daily via a single inhaler over LAMA/ICS/LABA free combinations. A large USA retrospective cohort study evaluated data for 1,821 patients with asthma taking ICS/LABA and who were prescribed add-on TIO.18 Approximately 16% of patients misinterpreted the instructions and switched over to TIO monotherapy.18 Of those patients who successfully started the intended open three-drug combination, 44% discontinued ICS/LABA within 6 months and continued on TIO monotherapy only.18 These real-world data demonstrated that prescribing drugs with different dosing instructions and in different inhalers can confuse patients and lead to dangerous noncompliance.
In summary, IND/GLY/MF showed comparable improvements in asthma-related quality of life and a lower rate of moderate exacerbations, which was significant at higher doses, compared to the open combination of SAL/FLU plus TIO. A greater reduction in symptoms was also seen with IND/GLY/MF by the end of the IRIDIUM study, reaching statistical significance for high-dose IND/GLY/MF. The safety profile was similar for both treatments. Prof Chapman concluded that the simplicity of the once-daily, single-inhaler, three-drug combination of IND/GLY/MF may be particularly beneficial in light of the real-world evidence, which shows a high risk of poor adherence with open combinations.
Could a New Device Improve Treatment Use and Adherence?
Professor Ken Chapman
The simplicity and intuitiveness of the Breezhaler device used to deliver IND/GLY/MF once daily in the PLATINUM study programme may also have the potential to address existing inhaler misuse issues and enhance correct use. Transparent capsules allow the patient to see that the full dose has been taken, therefore confirming complete inhalation of active medications.17,19 In a real-world study of patients with chronic obstructive pulmonary disease (COPD), those using the Breezhaler device made fewer critical errors compared to other commonly used inhalers.20 For example, just 15.4% of patients made one or more critical inhaler use error with the Breezhaler device, compared to 46.9% for Respimat® (Boehringer Ingelheim, Ingelheim, Germany).20
The Breezhaler device is also one of the lowest resistance asthma inhaler devices currently available. This allows patients to generate higher peak inspiratory flow rates as they inhale through it, which mobilises the medication for better deposition into the lower airway. In a randomised, open-label, multicentre Phase IV trial designed to compare peak inspiratory flow values generated by patients with COPD with moderate to very severe airflow obstructions, patients were able to inhale with the least effort through the Breezhaler device as compared to both Ellipta® (GlaxoSmithKline, Brentford, UK) and HandiHaler® (Boehringer Ingelheim, Ingelheim, Germany) dry powder inhalers.21
An exciting new advance with the Breezhaler device is the potential to monitor inhalation using the add-on proprietary Propeller® Sensor (Propeller Health, Madison, Wisconsin, USA). The sensor is attached to the Breezhaler device, which can then be linked to a smartphone app via Bluetooth, allowing patients to assess their own personalised asthma profile. Using the sensor allows patients to confirm that adequate inhalation has occurred and provides daily reminders to enhance medication adherence. Perhaps most importantly, the sensor for Breezhaler device also provides real-life use data that can be shared with physicians to support therapeutic decision-making in the clinic.
Could Digital Solutions Improve Virtual Consultations?
Professor David Price
The coronavirus disease (COVID-19) pandemic has highlighted the important role that virtual consultations can play in everyday clinical practice in asthma. Early evidence for the benefits of telemedicine can be seen in a 2003 study, in which patients requiring a routine asthma review were randomised to be offered a face-to-face consultation or a telehealth consultation. Of those offered a telehealth consultation, 74% used the service, whereas only 48% of those offered a face-to-face consultation used the service.22 Both patient satisfaction and quality of life outcomes were similar for those who received face-to-face care compared to a telehealth consultation.22
In recent months, COVID-19 has fuelled a huge expansion in telemedicine use around the world.23 This trend towards remote consultations has been broadly welcomed by patients and is likely to continue for the foreseeable future. Tools that can support HCP in navigating the new virtual landscape in asthma include digital portable spirometers and novel devices such as the Propeller Sensor.
Portable spirometers such as Air Next® (NuvoAir, Stockholm, Sweden) have proven to be as clinically valid and accurate as traditional physician-administered spirometers.24 They can be used to support diagnosis, reassessment, and self-monitoring of respiratory diseases by bringing objectivity into the treatment decision-making process. The Propeller system has also shown important benefits, including improved adherence, reduced emergency room utilisation, and a decrease in short-acting β-agonist use, which has highlighted its potential role in optimising asthma control. In two key studies of the Propeller system, controller medication adherence increased by 21% in 6 months, while asthma-related emergency room visits and hospitalisations were reduced by 57% over the course of 1 year.25,26 A further single-arm study of Propeller in nearly 500 patients with asthma found a substantial reduction in mean daily short-acting β-agonist use, which declined by 78% in 12 months.27
In summary, Prof Price concluded that the Breezhaler device has the potential both to address inhaler misuse and enhance correct use. Dosing is confirmed as patients can hear, feel, and see that the full dose has been taken. The Breezhaler device is also more likely to be used correctly because of its lower critical error rate compared to other devices and lower airflow resistance, which means inhalation is more comfortable for patients. In turn, the Propeller Sensor provides inhalation confirmation, medication reminders, and access to real-life data to support therapeutic decision-making, whether face-to-face or remote.
Concluding Remarks
Collectively, the data from the PLATINUM trial programme highlighted the clinical value of the two new once-daily combinations, IND/GLY/MF and IND/MF, delivered via the Breezhaler device.
IND/GLY/MF offers a fixed-dose combination of three key drugs with potential clinical benefits over classical standard-of-care for patients with inadequately controlled asthma. A once-daily regimen administered via a single inhaler may help improve convenience, treatment adherence, and control of both asthma symptoms and exacerbations.
The fixed-dose combination of IND/MF provides a once-daily dry powder option for patients whose asthma is poorly controlled despite treatment with ICS (with or without LABA). Once-daily IND/MF showed clinical benefit over other ICS/LABA and may be particularly convenient for patients who find a twice-daily regimen burdensome. Importantly, the availability of multiple dosing options for IND/MF ranging from low to high also provides clinicians with the scope to target and titrate dosing for individual patients as needed.