BACKGROUND AND AIMS
It is now accepted that sustained remission is the key treatment goal for rheumatoid arthritis (RA). The availability of biologic therapies and the use of targeted treatment strategies has led to increasing numbers of patients achieving remission; however, specific guidance for the management of patients with RA in sustained clinical remission, treated with conventional synthetic disease-modifying anti-rheumatic drugs (cs-DMARDs), is lacking.1-3 This raises concerns for over-treatment in asymptomatic patients. Tapering of treatment is encouraged, although there are no validated biomarkers predicting sustained remission.4,5 As such, there is an unmet need for predictors of sustained remission for tapering cs-DMARDs, which can be used in clinical practice.
The authors conducted a prospective observational study of two treatment strategies for patients with RA in stable remission. The primary objective was to assess the rate of sustained remission after 12 months, without flare in patients who were offered either structured cs-DMARD tapering or continued therapy. Secondary objectives were to determine baseline predictors of sustained remission following tapering and to develop a predictive model to help aid risk stratification of patients for tapering in clinical practice.
MATERIALS AND METHODS
Patients with RA in clinical remission (Disease Activity Score 28 for Rheumatoid Arthritis with C-reactive protein, [3v-DAS28CRP] <2.6) for ≥6 months on stable cs-DMARD therapy were sequentially recruited from a National Health Service (NHS), UK remission clinic. Patients were offered structured tapering, with 117 accepting tapering and 83 continuing therapy. Clinical, ultrasound (US), immunological (T-cell subsets), and patient-reported outcome (PRO) data were collected. The primary endpoint was the proportion of patients in each group in sustained remission without relapse after 12 months. Flare was defined as loss of DAS28 remission or evidence of at least one new clinically swollen joint. Logistic regression analyses were performed to identify predictors of sustained remission.
RESULTS
Two hundred patients fulfilled the inclusion criteria. No difference in baseline drug regimens (mono- versus combination therapy) was seen between the two groups. Male sex (p=0.036) and longer length of remission (p=0.015) were associated with the patient’s decision to taper, although not significantly after correction for multiple testing. The tapering group demonstrated lower levels of inflammation-related T-cells (IRC; p=0.001), inflammatory markers, and joint counts. No significant difference between groups was observed for PROs, although there was a trend towards lower PROs in the tapering group.
Of those who tapered, 64% remained in clinical remission after 12 months compared with 80% (p=0.018) of patients on stable therapy. In the tapering group, higher CRP, tender joint count, percentage of IRC, and higher PROs were associated with flare (all p<0.05), with a trend for higher total power doppler score on US (p=0.066). A multi-variable model (using clinical, US, PRO, and T-cell subset variables) predicting sustained remission (Figure 1) retained Rheumatoid Arthritis Quality of Life score (RAQoL), total power doppler score, and percentage IRC (85% accuracy; area under the curve of the receiver operating characteristic [AUROC]: 0.893; p<0.0001). A reference model using clinical variables only (as would be available in clinical practice) demonstrated only 69% accuracy (AUROC: 0.725). Other models, including combinations of two or three variables, demonstrated accuracies between 72–84% (Figure 1). In the non-tapering group, higher CRP, erythrocyte sedimentation rate, swollen joint count, and shorter disease duration (all p<0.05) were associated with flare, with no parameter able to predict sustained remission.
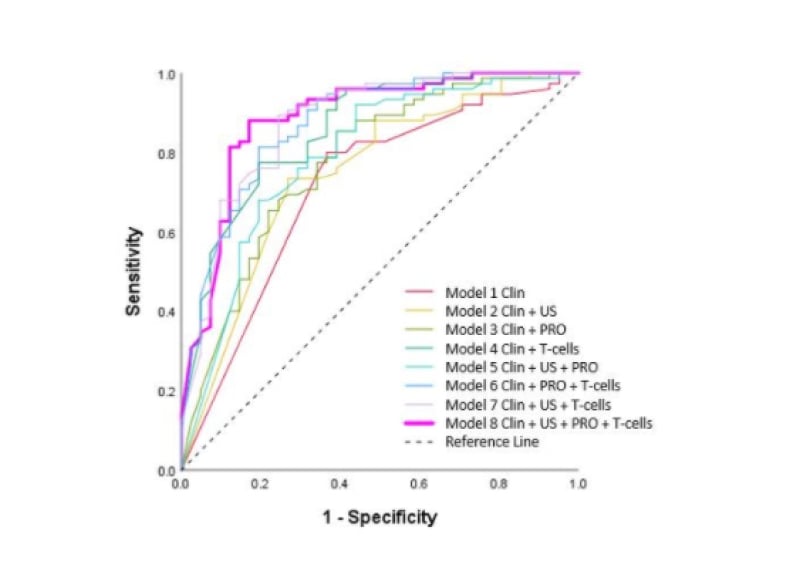
Figure 1: AUROC Models 1–8.
Clin: Clinical; PRO: patient-reported outcome; US: ultrasound.
CONCLUSION
The combination of clinical, PRO, US, and T-cell parameters demonstrated added value for predicting sustained remission compared with clinical parameters alone in the tapering group. These data could inform best tapering practice.








