Abstract
Medication-related osteonecrosis of the jaw (MRONJ) is a feared complication of anti-resorptive or anti-angiogenic therapy, presenting with non-healing areas of bone, which may form de novo or after dental intervention. The condition primarily affects patients under the care of oncologists and rheumatologists. Patients using these medications under the care of rheumatologists are predominantly being treated for osteoporosis, a highly prevalent condition causing considerable morbidity and mortality in the European population.
In the two decades since the condition was first described, there has been considerable progress in the understanding of the pathophysiology of the condition, although this remains incomplete. Additionally, clinicians may now benefit from long-term follow-up data to give a more evidence-based approach to MRONJ risk stratification. At present, there is considerable variation between guidelines produced by advisory groups. This paper focuses exclusively on the osteoporotic cohort, and aims to review recent findings to explore the differences in risk profiles between osteoporotic and oncological cohorts, as well as between different anti-resorptive medications. Further sections discuss prevention and management of MRONJ in osteoporosis, including the timing of tooth extraction, and consider the direction of future research. The findings suggest that patients with osteoporosis treated with bisphosphonates carry an extremely low risk of MRONJ, although denosumab presents a higher risk. Nevertheless, the reduced fracture rate from prompt treatment with anti-resorptives likely outweighs the risk of MRONJ. Dental hygiene should be optimised to reduce risk, and tooth extraction should take place in a timely fashion, with no convincing evidence to support the use of drug holidays. Treatment at present favours a surgical approach, with potential roles for antibiotics, but at present there is insufficient evidence for other medical adjuncts.
Key Points
1. Patients with osteoporosis should be recognised as being at considerably reduced risk of medication-related osteonecrosis of the jaw (MRONJ) relative to oncology patients, and risk stratified accordingly to avoid unnecessary delays to treatment.
2. The route and duration of antiresorptive administration appears not to significantly affect the risk of MRONJ, but there is a dose-dependent risk, which does not appear to be reduced by a drug holiday.
3. Tooth extraction is recommended if required, without delay. Higher risk patients should have extraction performed under the care of a maxillofacial service, and all patients with established MRONJ should also be referred.
INTRODUCTION
Osteoporosis is a common disease, with a mean prevalence of 5.6% within European (EU27+2) countries, and an annual cost to these countries of EUR 56.9 billion.1 This figure is illustrative of the significant morbidity and mortality burden associated with these fractures. As the population of Western Europe ages, preventing and treating this often debilitating condition continues to gain ever-greater importance.
It has become apparent, however, that treatment or prophylaxis of osteoporosis may be associated with medication-related osteonecrosis of the jaw (MRONJ), predominantly occurring in patients with cancer or osteoporosis, as a rare but debilitating side-effect of anti-resorptive or anti-angiogenic therapy, although the latter group is beyond the scope of this paper. MRONJ is a relatively recently described phenomenon, first appearing in the medical literature in 2003 as part of a case series from Marx et al.2 Since the initial description, there have been considerable advances in the understanding of the condition, which may inform changes to current practice for clinicians involved in the care of patients with osteoporosis. The condition is most frequently described in patients in oncology and osteoporosis. This paper aims to highlight the changes in evidence impacting current clinical practice in the prevention and management of MRONJ in relation to osteoporosis, as the two groups have different risk profiles, and the approach to osteoporosis is often conflated with that for the oncology population.
The first cases described were in association with nitrogen-containing bisphosphonates, which act to inhibit the mevalonate kinase pathway to induce osteoclast apoptosis. Bisphosphonates remain the most commonly reported cause of MRONJ. The disease occurs preferentially in the mandible rather than the maxilla, at a ratio of approximately 2:1, with 9% of cases having involvement at both sites.3 Since the inaugural paper, further definitive associations have been made with other anti-resorptive medications, particularly the receptor activator of nuclear factor κ-B ligand inhibitor denosumab, but also the newer anti-sclerostin agent, romosozumab.4,5
The American Academy of Oral and Maxillofacial Surgeons (AAOMS) have set widely accepted diagnostic criteria for MRONJ, which needs to fulfil all three of the following criteria to make a diagnosis:6 current or previous use of anti-resorptive therapy alone, or in combination with immune modulators or anti-angiogenic medications; exposed bone in the maxillofacial region persisting for more than 8 weeks, either visualised directly, or discovered via probing oral fistulae; and the absence of significant radiation exposure or metastatic disease of the affected area.
The clinical course is variable, and many patients remain asymptomatic for prolonged periods. However, pain; gingival inflammation, ulceration, and fistulation; bony enlargement; tooth loosening; and secondary osteomyelitis may occur.7 Treatment is extremely challenging and may involve both medical and surgical approaches. Prevalence is highest in the oncology population, thought to be a dose-dependent result of the high cumulative dosages used to limit bony destruction or control malignant hypercalcaemia, with typical dosing regimens resulting in administration of doses 12–15 times higher per annum than those used in osteoporosis.8 These drugs are also widely used within rheumatology services for metabolic bone disease, most frequently in the case of primary osteoporosis, but also for the purpose of treatment or prophylaxis of secondary osteoporosis, typically where prolonged treatment with steroid therapy is required. Anti-resorptives also find usage in rarer metabolic bone disease such as Paget’s disease of bone, fibrous dysplasia, and osteogenesis imperfecta. Thus, consideration of risk of MRONJ presents a frequent challenge for the rheumatologist, and requires interdisciplinary collaboration with maxillofacial and dental colleagues.
IDENTIFICATION AND MANAGEMENT OF HIGH-RISK PATIENTS
Risk Stratification
Recent evidence, collated in the 2022 AAOMS update, suggests that, as a consequence of the high estimated MRONJ prevalence of 1–17% in the oncology population, the risks of MRONJ may have received undue prominence in patients receiving the medication for other indications.6,9 This may have come at the cost of an increased number of fragility fractures in the face of patient and physician reluctance to use anti-resorptives in a timely fashion on this basis. The low prevalence of MRONJ should be contrasted with the results from a large-scale meta-analysis performed by Crandall et al.,10 which indicated a number needed to treat of between 30–89 for denosumab and bisphosphonates over the first 1–3 years of treatment, depending on fracture site and gender. Although the number needed to treat may appear relatively high, the high prevalence and severe morbidity and mortality of osteoporotic fracture must be taken into account. If osteoporotic treatment is delayed by screening and assessment, there is considerable risk of subsequent preventable fractures.
Increasing duration of therapy has been shown to correlate significantly with incidence of MRONJ in oncology patients. However, the data for osteoporotic patients is less clear. Initial support was found from a paper undertaking retrospective case identification via a postal questionnaire, demonstrating an increase from 0.00% prevalence at baseline to 0.21% prevalence at 4 years for patients on oral bisphosphonates. Subsequent prospective controlled cohort studies failed to demonstrate the same findings, although it should be noted that these were neither designed nor powered to assess MRONJ cases.6 Both bisphosphonates and denosumab now benefit from long-term follow-up data, enabling accurate assessment of prevalence, which is challenging existing assumptions. The prevalence of MRONJ in patients exposed to bisphosphonate ranges from 0.02–0.05%, with zoledronate showing no higher risk than oral bisphosphonates. Denosumab demonstrates a 10-year prevalence of 0.30%, and the current emerging data on romosozumab suggest that there is a prevalence of 0.02–0.03%.6 Given that many guidelines continue to consider intravenous bisphosphonates to be higher risk, this suggests that dose, rather than route, is the differentiating factor.
Nevertheless, patients with osteoporosis are not a homogenous group. While the majority of those treated will have primary osteoporosis, patients with osteoporosis related to a rheumatic inflammatory disease (either the disease itself or the treatment thereof, e.g., where prolonged treatment with steroids is required) may be at higher risk of MRONJ, with one study demonstrating a 1.5% prevalence of MRONJ in this group (n=198).11 This has a plausible mechanism; rheumatic inflammatory diseases, particularly rheumatoid arthritis, are known to be associated with MRONJ risk factors such as periodontitis.9 Glucocorticoids are well known to be a cause of osteoporosis, but are also a risk factor for MRONJ. As part of the wide-ranging effect of administration of glucocorticoids, osteoporosis is thought to relate not only to induction of osteoblast apoptosis, but also to inhibition of osteoclast function via a separate pathway to each anti-resorptive, compounding existing issues of reduced bone turnover.12 This also applies to another putative mechanism of MRONJ, reduced angiogenesis. Glucocorticoids have also been shown to reduce vascular endothelial growth factor expression, again amplifying anti-angiogenic effects of bisphosphonates and denosumab, which have been demonstrated in vivo through murine models, contributing to emerging necrosis.13,14 Larger multicentre studies would be required to more definitively evaluate the influences of rheumatic inflammatory disease and the medications used to treat them, which may influence stratification of non-oncological patients.
Finally, poor oral health at the time of commencing therapy is a key risk factor for MRONJ. A 2005 paper from Marx et al.15 demonstrated, from a sample of 119 patients with MRONJ, a considerably higher prevalence of periodontitis (84.0%), caries (28.6%), and dental abscess (13.4%) than the general population. Tooth extraction has classically been considered the key risk factor for MRONJ, but again, the osteoporotic population are at considerably lower risk, with a meta-analysis by Gaudin et al.16 demonstrating a 0.15% rate of MRONJ (p=<0.0001) in this cohort after tooth extraction. While no comparison study for conservative and surgical strategies has been performed in osteoporotic patients, a dual-centre study of 189 oncology patients demonstrated a dramatic difference in MRONJ development after propensity matching. Those in whom tooth extraction was avoided demonstrated approximate rates of 90% MRONJ occurrence by 8 years, but those who underwent tooth extraction displayed rates of <20%, although all cases in the latter group occurred within 2 years.17 This would support the hypothesis that local inflammation or infection is a predominant driver of MRONJ, and the requirement for extraction is a symptom of the conditions favouring MRONJ, rather than the direct cause. This view is supported by the observation that pre-existing periodontal or periapical disease without any oral intervention/trauma is sufficient to cause spontaneous MRONJ in approximately 25% of identified patients,18,19 as well as the relatively minor trauma caused by ill-fitting removable dentures, especially at the retromylohyoid fossa.19
Prevention of Medication-Related Osteonecrosis of the Jaw
The field currently suffers from a lack of high-quality studies in order to assess the benefit of preventative procedures. A Cochrane review from 2017, subsequently updated in 2022, found insufficient evidence to support the conclusion that any studied prophylactic or therapeutic intervention is of benefit in MRONJ.20 But, as tooth extraction and periodontal disease are the most common risk factors for developing MRONJ, prevention is predominantly targeted towards optimising oral health and modulating modifiable dental (e.g., extraction versus root-retentive treatment) or medical risk factors (e.g., review of anti-resorptive/anti-angiogenic and corticosteroid treatment).21
Routine screening of at-risk patients is recommended across several international consensus statements on the prevention and management of MRONJ.6,22,23 The Scottish Dental Clinical Effectiveness Programme (SDCEP) recently updated their National Institute for Health and Care Excellence (NICE)-accredited guidance in the oral health management of patients at risk of MRONJ to stratify patients into low and high-risk groups.24 The high affinity of bisphosphonates to hydroxyapatite results in a persistent dose-dependent effect that can last up to 10 years, whereas denosumab is cleared through the reticuloendothelial system with a half-life of approximately 26 days.25,26 This is reflected in the SDCEP guidance, which stratifies low-risk patients as those taking denosumab for any length of time, whereas patients on oral or intravenous bisphosphonate become high-risk with over 5 years of use.24 Indeed, after 9 months without denosumab, SDCEP classify the patient as having no risk of MRONJ.
Some authors advocate an aggressive approach to maintaining oral health in at-risk patients, with 5,000 ppm fluoride toothpaste and overnight fluoride gel bathing of equivocal prognosis dentition.27 Following on from this, dental treatment, including dentoalveolar surgery, should proceed as normal for all patients, with the caveat of aiming for root retentive treatment in high-risk patients if possible, although protocols remain unstandardised.28-30 A non-healing extraction site of over 8 weeks necessitates maxillofacial referral. The role of peri-/post-exodontia antibiotics in preventing MRONJ is controversial. In a systematic review by Cabras et al.,31 only one out of 17 studies found a higher risk of MRONJ without antibiotics.
So-called ‘drug holidays’, referring to temporary discontinuation of bisphosphonates, remain a contentious issue regarding their benefit in either prevention or treatment of MRONJ. No consensus exists in adjudicating the balance between the risk of osteoporotic fractures with that of developing MRONJ. Patients using denosumab should not undertake drug holidays, as they are at increased risk of vertebral fractures if the drug is stopped: a post hoc analysis of the FREEDOM trial demonstrated an increased multilevel vertebral fracture rate that was apparent within 3 months after omission of a scheduled dose.32
A recent systematic review did not show any evidence for a bisphosphonate holiday in MRONJ.33 Given the excess mortality of hip fracture at 1 year is up to 36%, the benefit of fracture prevention likely outweighs the low risk of MRONJ, and should be assessed using the Fracture Risk Assessment (FRAX) tool to help guide clinical decision making.34,35 A reasonable compromise in patients taking denosumab, given the half-life and apparent increased risk relative to other anti-resorptives, is to plan dentoalveolar surgery for 3–4 months after the last denosumab dose, and resume 6–8 weeks post-surgery.6
It is important to acknowledge, however, that drug holidays remain appropriate for risk reduction of other complications associated with bisphosphonate therapy. A large retrospective Swedish study demonstrated a 70% annual reduction in adjusted odds ratio of atypical femoral fracture in bisphosphonate users since drug cessation.36
MANAGING PREVENTION OF MEDICATION-RELATED OSTEONECROSIS OF THE JAW
At present, it is inevitable that a small proportion of patients treated with anti-resorptives will go on to develop MRONJ. Management strategies for established disease are also required, which may be operative or non-operative in nature. If the patient has not already been referred to a maxillofacial team, this should, of course, be performed as a matter of urgency. Non-operative or operative strategies may be pursued at any point within the stages of MRONJ, depending on AAOMS staging (Table 1).
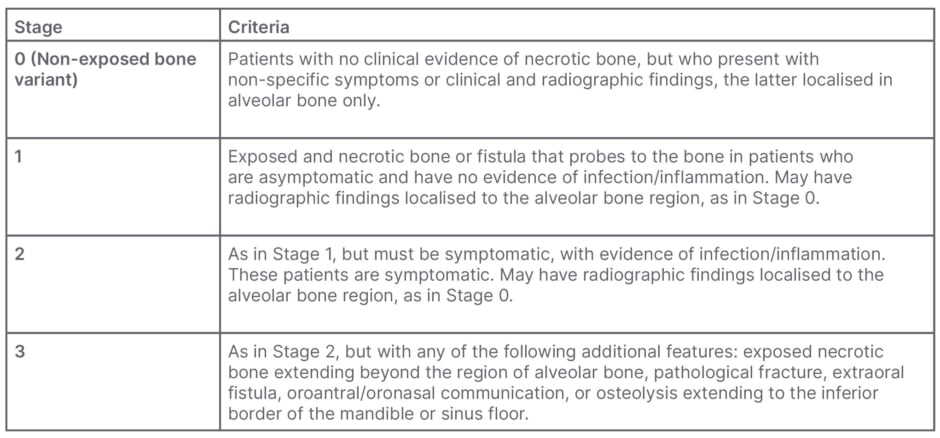
Table 1: Summary of American Academy of Oral and Maxillofacial Surgeons (AAOMS) staging criteria for medication-related osteonecrosis of the jaw severity.6
Certain measures are appropriate for all stages of MRONJ. All patients should receive education aiming to explain the slow rates of improvement and resolution over a period of months to years, and the intended aims of treatment, particularly symptom improvement and pain control. A cornerstone of therapy is improved oral hygiene, which may help those Stage 0 patients who will progress to the exposed bone variant, with one case series demonstrating a progression rate of 53.1%.37 Mobile or well-formed bony sequestra should also be removed as a potential nidus for infection. Chlorhexidine solution should be used in all patients with established MRONJ, and may well prove sufficient for cure in Stage 1 patients when used as part of a local wound care strategy, aiming to disrupt the biofilm surrounding the necrotic bone and prevent progression of disease accordingly.38 In Stage 2 or 3 disease, antibiotics and analgesia may be added.6
The rationale for antibiotic therapy relates to the key micro-organism group within the biofilm, Actinomyces spp. These facultative anaerobes are now thought to play a role in the pathogenesis of MRONJ, rather than just being a superficial contaminant. In a retrospective cohort study, Russmueller et al.39 detected Actinomyces spp. in 89% of histologically confirmed MRONJ cases. β-lactam antimicrobials remain the agents of choice, with tetracyclines being an acceptable alternative in patients with penicillin allergy as Actinomyces spp. isolates have been shown to be almost uniformly resistant to metronidazole, and thus should be avoided.39,40 Although there is a debate regarding the timing of antimicrobials prior to surgery, high dose β-lactam antimicrobials in the days prior to surgery appears to be a reasonable strategy.39
Excellent results have been reported for surgical intervention, which may dramatically enhance resolution/improvement rates in comparison to conservative strategies.6 The decision on when to undertake operative treatment should not be based solely on the clinical or radiological stage of disease, but also on the projected impact on quality of life, and capacity of the patient to undergo challenging bony and soft tissue reconstruction. Surgical options typically include initial debridement, saucerisation, or sequestrectomy in Stage 1 disease. The extent of mandibular or maxillary bony resection in Stage 1 and 2 disease largely depends on the height of disease-free alveolar bone available, and extent of disease in relation to the inferior alveolar nerve canal or maxillary sinus.6 By definition, Stage 3 surgical management necessitates segmental resection or partial maxillectomy and appropriate reconstruction, but full discussion of reconstruction is beyond the scope of this article.
There is evidence to suggest early sequestrectomy and primary mucosal closure in Stage 1 disease can halt disease progression and even downstage lesions.41,42 Vescovi et al.43 have shown that conservative surgical interventions should be considered in patients unresponsive to 6 months of non-invasive therapy. In advanced disease, the controversy lies in when to surgically intervene. Debate still exists as to whether a period of non-operative therapy is beneficial in stabilising disease, as recommended in the AAOMS treatment algorithm, or whether aggressive primary surgery results in a shorter time to achieving restoration of mucosal integrity.6 A recent systematic review compared surgical treatment options in Stage 3 disease.44 With primary outcome measures, including full mucosal healing and disease downstaging, marginal resection without microvascular flap reconstruction resulted in a full mucosal healing rate of 85% compared with 54% with sequestrectomy alone. The addition of microvascular flap reconstruction resulted in a mucosal healing rate of 97%, likely due to the additional benefit of a segmental resection in more thoroughly removing all non-vital tissue. The success rates seen provide a firm mandate for surgical management being the mainstay of therapy at present.
A number of strategies have been trialled to improve non-operative treatment options. As with prophylaxis, no clear benefit to a drug holiday of any anti-resorptive has been observed, and the majority of studies favour lack of benefit. While the prolonged half-life of bisphosphonates supports these results, the shorter half-life of denosumab, at just 26 days, would suggest a benefit to withdrawal.45 However, in order to avoid rebound bone loss at discontinuation, a separate agent would need to be implemented. In the absence of MRONJ, denosumab cessation is now typically accompanied by zoledronate to maintain bone density gains from the period of denosumab therapy.46 In a patient with established MRONJ, it is currently assumed that adding anti-resorptive therapy would be counterproductive. Withdrawal of anti-resorptives after confirmation of MRONJ, therefore, cannot be recommended at this stage in time, although further evidence may emerge, particularly surrounding romosozumab.
One therapy that has shown particular promise in improving MRONJ resolution rates is teriparatide, with Kim et al.47 noting a statistically significant difference in the percentage of patients achieving resolution or improvement and also in rate of change over a 6-month period, albeit within a retrospective design. A further study suggested equivalence of weekly and daily injections, although with just one patient in each arm.48 A placebo-controlled, prospective, randomised controlled trial of teriparatide failed to demonstrate statistical significance, which may have related either to the short duration of therapy of just 8 weeks, or to the small study size (n=34).49 It would be tempting to consider the possibility of initiating teriparatide to continue treating both the MRONJ and osteoporosis and allow for denosumab cessation, but the DATA-Switch trial has provided evidence that teriparatide alone is insufficient to prevent bone loss after cessation of denosumab.50 A high-quality prospective, randomised controlled trial, ideally with both weekly and daily administration arms, is required before teriparatide can be recommended as an integral part of medical management of MRONJ. After initial medical and surgical strategies have been implemented, patients must remain under close follow-up in order to assess response. At present, clinical history and oral examination, coupled with radiographic surveillance, are the mainstay of this process. There has been some interest in the use of bone turnover markers to predict recovery, which have proved disappointing in predicting risk of MRONJ, where the majority of research attention has been focused.51 One retrospective study found a statistically significant difference between the levels of bone turnover biomarkers of serum osteocalcin, C telopeptide, and bone alkaline phosphatase in patients who recovered and those who did not. However, the results should be interpreted with caution, given the trial design and absence of documentation of potentially confounding issues such as bony metastases, a particularly pertinent issue given the high numbers of oncology patients included.52 Nevertheless, a sensitive and specific biomarker would be of great utility in guiding the ongoing management of these patients.
CONCLUSION AND KEY POINTS
Over the last 20 years, significant progress has been made in the understanding of the underlying pathophysiology of MRONJ. Nevertheless, our mechanistic understanding remains incomplete, and many clinical guidelines have not been recently updated to reflect the increased body of evidence available. As such, the authors would make the following suggestions for patients with osteoporosis:
• Unnecessary delays for routine dental review before starting bisphosphonates are likely to worsen outcomes, but regular dental review is important to optimise oral health and prevent MRONJ.
• The route and duration of anti-resorptive administration appears not to significantly affect the risk of MRONJ, but there is a dose-dependent risk.
• Tooth extraction is recommended if required, without delay. Higher risk patients should have extraction performed under the care of a maxillofacial service.
• There is limited evidence to support drug holidays for the purpose of decreasing MRONJ risk, but they do reduce the risk of atypical femoral fracture.
• Further research to stratify the risk of MRONJ within the osteoporotic cohort through study of rheumatic inflammatory diseases and drugs used to treat them would be highly beneficial.
• Surgical management remains the cornerstone of therapy. Antibiotics are the only medical adjunct with convincing evidence of benefit in MRONJ at present.







