Abstract
Ankylosing spondylitis is a chronic autoimmune inflammatory condition belonging to the spondyloarthropathy category of rheumatic diseases. It typically affects the axial skeleton but may also present with peripheral arthritis and extra-articular features. Ankylosing spondylitis tends to occur in patients under the age of 45 years, has a higher incidence in males, and can lead to disability and reduced quality of life if not adequately treated. Management consists of a multidisciplinary team approach. Although traditional disease modifying anti-rheumatic drugs are less effective for the axial component of this disease, biologic therapies do seem effective. In severe cases, surgery may be warranted.
INTRODUCTION
Ankylosing spondylitis (AS) has been afflicting humankind as far back as ancient Egypt.1,2 It was during the 1800s that the classical description of AS was made.3 Throughout the 1900s, further understanding about the disease was established, including its hereditary nature.3 The disease is recognised as part of the spondyloarthropathy group of rheumatic diseases. These include psoriatic arthritis, reactive arthritis, and arthritis associated with inflammatory bowel disease.2 These conditions share similar clinical features and an association with human leukocyte antigen (HLA)-B27.
The primary sites of inflammation in AS are the sacroiliac joints. Males tend to be more commonly affected than females,2 although studies over the years have shown this sex difference to be a lot more marginal than was initially thought.3 It primarily affects young adults, with a higher incidence in patients younger than 45 years old. As the disease progresses it can result in total fusion of the axial skeleton, and can cause loss of physical function and spinal mobility.4 Patients in which the disease has been inadequately treated or undiagnosed can develop a characteristic ‘bamboo spine’ where there is total spinal fusion. As well as chronic pain, this can also result in restrictive lung function, leading to respiratory failure.2
AS is not just limited to the spine; the peripheral joints can be affected, and organs such as the eyes, heart, and lungs can be involved. Patients can also complain of systemic symptoms such as fatigue or weight loss. There is a high risk of osteoporosis and vertebral fractures.3 Chronic pain and immobility can lead to patients experiencing depression and anxiety. There is a socio-economic burden as patients may be unable to work, either due to their symptoms or a workplace that may not be adapted for people with arthritis. Thus, it is important to recognise that this is a multisystem disease and the clinician should be wary of focussing purely on spinal symptoms.
Definite diagnosis can be delayed as radiographic changes of sacroiliitis occur late in the disease process.3 It can take up to a decade for radiographic changes to appear, with a proportion of patients never going on to develop radiographic changes at all. Patients who present without radiographic changes are described as having non-radiographic axial spondyloarthritis (nr-axSpA), with debate in the literature over whether this represents a separate disease entity altogether.3,5 Classification criteria have been updated to include the diagnosis of nr-axSpA. It should be noted that certain treatments, such as infliximab and interleukin (IL)-17 inhibitors, are not approved for nr-axSpA according to the Assessment of SpondyloArthritis international Society-European League Against Rheumatism (ASAS-EULAR) 2016 recommendations, but other biologic drugs are approved.5 Overall, however, it is argued that only the single term of AS should be used to encompass the disease.
PATHOPHYSIOLOGY
Thanks to advances in imaging techniques, clinicians have a better understanding of how AS results in structural damage to the axial skeleton. While the underlying mechanism triggering the inflammatory process is unknown, study of the pathology of AS has revealed cells that are involved in the process.
HLA-B27, a Class 1 surface antigen, is found in ≤89% of AS patients,3 and is strongly associated with the spondyloarthropathy group of diseases.2 HLA-B27 binds antigenic peptides for presentation to cytotoxic T cells, thus enabling normal function of the immune response in targeting pathogens such as the influenza virus.6 The exact mechanism by which HLA-B27 plays a role in AS is so far unexplained. Nevertheless, it is thought to involve abnormalities in antigen presenting cells, subsequently triggering the inflammatory cascade.3
There has been interest in the enzyme ERAP1 and how this may be linked to the pathophysiology of AS.7 The enzyme’s function is to trim peptides for binding to HLA Class 1 molecules.7 It has been hypothesised that ERAP1 exerts a pathogenic effect by altering the interaction between HLA-B27 and immune receptors.7 Further research is being conducted into this area of AS to ascertain whether ERAP1 could provide a therapeutic target.
Whatever the initial events, AS results in structural damage to the axial skeleton. Proinflammatory cytokines, such as IL-17 and tumour necrosis factor (TNF)-alpha, are released, which activate cells causing bony destruction. Another cytokine, IL-22, causes osteoproliferation. These processes can sometimes occur simultaneously.3 As a consequence of new bone growth, syndesmophytes develop inside ligaments,8 which are considered a hallmark radiological feature of AS. At its most severe, this process can lead to complete fusion of the axial skeleton.3
TNF-alpha has been found to be elevated in the serum and synovial tissue of patients with AS,4 and has been an important therapeutic target. TNF inhibitors have been shown to be effective in reducing disease activity and stiffness;4 thus suggesting an important role for this cytokine in the pathogenesis of AS. There is still debate, however, as to whether TNF inhibitors are truly disease modifying in AS.8 The recognition of IL-17 and IL-23 in the pathogenesis of AS has enabled researchers to develop further therapeutic options.
CLINICAL FEATURES
Back pain is a common symptom that most people experience at one time or another during their lives. There are many different causes of back pain and, therefore, clinicians need to be aware of how to distinguish symptoms characteristic of inflammation from other causes. Inflammatory back pain characteristically improves upon activity and worsens with rest. It should be present for at least 3 months to warrant further investigation.3 The pain is described as dull and insidious in onset and may be nocturnal, interfering with the patient’s sleep pattern. Early morning stiffness lasting longer than 30 minutes is an important feature. Patients commonly complain of lower back or buttock pain.3 This could be unilateral initially but become bilateral as the disease progresses.2 The cervical and thoracic spine can also be affected but less commonly. Table 1 summarises the characteristic features of inflammatory back pain.2,9
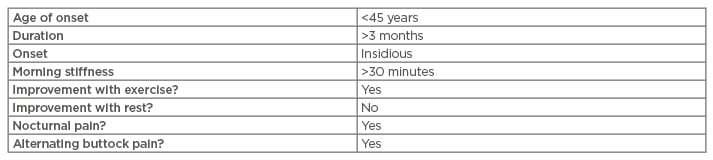
Table 1: Characteristic features of inflammatory back pain.
AS typically presents in patients under the age of 45 years and is more prevalent in males, although it can occur in female patients too.2 The pain may progressively worsen. Neurological symptoms can occur in AS secondary to spinal fractures. If a patient complains of new onset back or neck pain following injury with a background of severe AS, the patient should be investigated for fractures.3 Clinicians should be vigilant of the possibility for osteoporosis in these patients and treat as appropriate.
Psoriasis and inflammatory bowel disease can occur secondary to AS.3 Other extra-articular manifestations include anterior uveitis and peripheral arthritis.2,3 The joints most commonly affected are the large joints of the knees, hips, and shoulders.2 Hip disease in particular can be very disabling and some patients may require a total hip replacement if there is structural damage.2 Enthesitis and dactylitis can be a presenting feature.3 Aortic incompetence, upper lobe pulmonary fibrosis, and renal disease secondary to amyloid deposition can occur in AS.2 Some studies have also suggested AS patients are at an increased risk of nephrolithiasis when compared with the rest of the population.10 Other cardiovascular abnormalities, such as conduction defects, can be present and can have important haemodynamic consequences.11 Thus, patients should be screened for any cardiovascular and respiratory symptoms when seen in the clinic. Systemic features such as fatigue, weight loss, and low-grade fevers could be present, indicating an inflammatory process.
On examination, patients can display evidence of spinal deformity, including loss of normal lumbar lordosis and kyphosis. The sacroiliac joints may be tender to touch and there may be restriction of normal movement of the lumbar and cervical spine, along with reduced chest expansion. The Schober’s test is used to measure lumbar flexion; <5 cm of flexion is considered an abnormal result. Patients may have swollen and tender peripheral joints in an asymmetrical distribution. There may also be tender enthesial points, such as the Achilles tendon insertion.
INVESTIGATIONS
Laboratory abnormalities, such as elevated inflammatory markers and normocytic anaemia, are present in most cases, but in some cases patient’s blood tests can be normal.12 An elevated C-reactive protein may only be found in 50% of cases. However, it is useful to request a C-reactive protein test as, if elevated, it can indicate a favourable response to biologic therapy and can help to differentiate from mechanical causes of back pain. HLA-B27 is sometimes requested to help aid diagnosis, although in clinical practice some clinicians do not routinely ask for this test as it can be present in up to 7% of the Caucasian population. Renal function should also be monitored.2
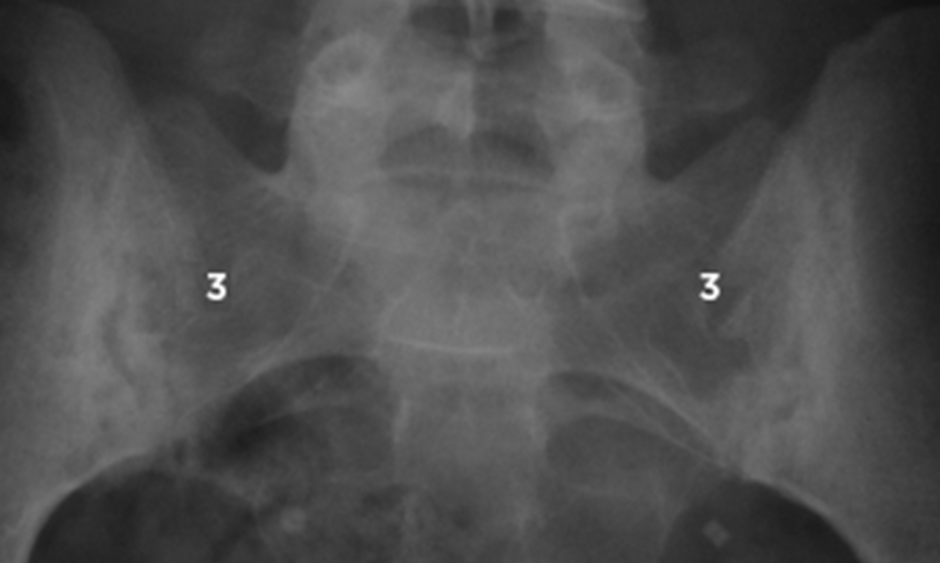
Plain radiographs of the sacroiliac joints can show characteristic changes of sacroiliitis. The joint margins can look blurred, with erosions and loss of joint space (Figure 1).12 In advanced cases, the joints may be completely fused. Sacroiliitis can be graded radiographically from 1–4, according to the modified New York criteria (Table 2).13 Plain films of the spine may show vertebral body squaring along with syndesmophytes.12 Erosions may also be present. The bones may demonstrate radiological osteopenia, similar to other inflammatory conditions. Radiographs may also pick up vertebral fractures.
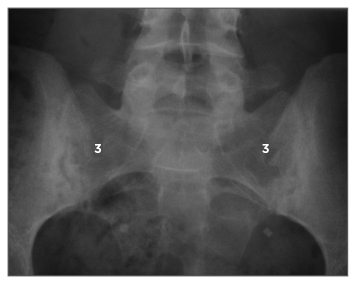
Figure 1: Bilateral sacroiliitis on X-ray.
3: Grade 3 sacroiliitis-erosions and sclerosis at the sacroiliac joints.
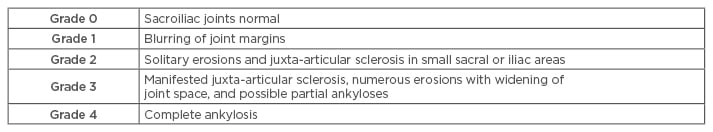
Table 2: Sacroiliitis grading on plain film.
In the early stages of the disease, plain films may be normal.3 There is also variation in interpreting plain films, which can cause uncertainty around reaching a diagnosis.3 Therefore, magnetic resonance imaging (MRI) may be required, as it has been shown to be more sensitive in detecting inflammation than plain films or computed tomography (CT) scans.3,12 Indeed, in the early stages of the disease, MRI will show inflammation whilst plain films can be normal.5 An MRI should be requested in those patients who complain of inflammatory back pain but have normal plain radiographs. As well as showing spondylitis, erosions, and arthritis, MRI can also demonstrate enthesitis, which is not evident on plain films. Bone marrow oedema indicative of localised inflammation can also be revealed by MRI.14 It can be used to objectively monitor disease activity beyond patient and physician reporting. Table 3 highlights active and chronic inflammatory lesions as seen on MRI according to the ASAS criteria.15 Active changes are best visualised on short tau inversion recovery sequence. Chronic lesions are best seen by a T1-weighted sequence.15
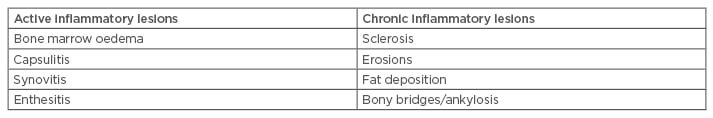
Table 3: Types of magnetic resonance imaging lesions in ankylosing spondylitis.
TREATMENT
Management of AS can be challenging. Conventional treatments for inflammatory arthritis lack evidence for efficacy in AS.5 Most patients are young and are in work; AS can be debilitating and cause considerable socio-economic burden because patients have to take time off work and, in some cases, may have to leave their job altogether. Having a chronic illness can also be associated with depression and anxiety. Counselling and psychological support may be needed if the patient is suffering from depression or low mood secondary to their illness. Patient education is an important part of management.2 Patients should be given leaflets on their condition and be directed to support groups, which can provide them with help and further information if needed. Patients with a greater understanding of their disease are more likely to be compliant with their management.2
The ASAS-EULAR group has recently updated its recommendations for the management of AS.5 It recommends that AS be managed in a multidisciplinary setting, with due attention to both pharmacological and non-pharmacological treatments that take into account the societal and psychological costs of AS. Patients should complete questionnaires at every appointment to assess their pain levels, functional abilities, and quality of life. Inflammatory markers can be useful to help assess levels of disease activity and act as a useful guide to the likelihood of improvement with TNF inhibitors. Repeat imaging of the spine should only be conducted if necessary, as the information gleaned may be limited due to the slow rate of radiographic progression. If they are to be repeated then the ASAS-EULAR group recommends an interval of at least 2 years between radiographs.5
Non-pharmacological management includes advising the patient on lifestyle measures. Prompt referral to physiotherapy is essential, and patients should be encouraged to attend therapy appointments because exercise programmes are beneficial in maintaining patients’ mobility and posture. They should keep up with exercise even when established on drug treatment.2 Hydrotherapy can be recommended for patients who suffer from widespread body pain and stiffness. Stopping smoking may also be advisable, as there is a possible association between disease activity and smoking.16 If available, occupational therapy should be offered so patients can receive support with activities of daily living and any workplace modifications that may be needed.
Non-steroidal anti-inflammatory drugs (NSAID) are the first-line recommended agents for use in AS. One study examined the proportion of AS patients responding to NSAID after 4 weeks of treatment at the maximal tolerated dose. The study found that the majority of active AS patients benefitted from NSAID.17 There was no difference in response between patients labelled as having non-radiographic AS when compared with radiographic AS. However, 44% of patients at the end of the study still had high disease scores and thus qualified for treatment escalation. Long term use of NSAID also has adverse effects, which include gastrointestinal upset, hypertension, and renal disease, thus limiting their use. In addition to NSAID, other analgesics, such as opioids, can be prescribed depending on patient and clinician preference.
If needed, local intra-articular steroid injections into the sacroiliac and peripheral joints can offer relief. However, systemic glucocorticoid treatment has not been proven to be as efficacious for AS when compared to other inflammatory conditions. Long term steroid use can also contribute to osteoporosis.2 CT-guided sacroiliac joint injections have been shown to provide effective pain relief for up to 6 months provided correct positioning of the needle tip is used.18 Clinicians should be aware of the risk of tendon rupture with local steroid injections for enthesitis and these should never be used around the Achilles tendon insertion.
Disease-modifying anti-rheumatic drugs, such as methotrexate and leflunomide, are not routinely used in AS due to lack of evidence for efficacy.5,19 Methotrexate, at a high dose of 20 mg subcutaneously, was not shown to improve patients with axial symptoms in one study.19 Evidence for its use in patients with peripheral arthritis due to AS still requires further study. However, there is evidence to suggest that sulfasalazine can be beneficial if patients are suffering from peripheral arthritis and early morning stiffness.20 Patients should be counselled about the potential side effects, such as neutropenia and drug-induced lupus. Family planning also needs to be taken into consideration, as treatment can cause low sperm counts, although this is reversible upon drug cessation.
If patients have persistently high disease activity despite the above therapeutic options, then the next step would be treatment with biologic agents which include TNF inhibitors. The agents currently recommended are adalimumab, certolizumab pegol, etanercept, golimumab, and infliximab, and their biosimilars. There is good evidence to support the use of biologics in AS.3 Spinal inflammation, as detected by MRI, has been shown to reduce after anti-TNF treatment.12 Up to 60% of patients have a good response to these agents. Patients report reduced pain scores and better physical activity with biologic treatment and are generally well tolerated.4 Patients should be screened for hepatitis, HIV, and tuberculosis prior to starting treatment. Once started, a patient should be assessed after 12 weeks for treatment efficacy. If there has been no improvement or the patient is unable to tolerate the drug, then they can be switched to a second biologic agent. Side effects such as neutropenia and nausea can occur, along with drug-induced lupus, although uncommon. Congestive cardiac failure is a relative contraindication to these drugs. Occasionally, treatment may have to be interrupted if a patient suffers from an infection or undergoes surgery, and once recommenced may not be as effective. While there is no particularly preferred biologic agent to use in first-line therapy, it should be noted that etanercept is not effective in treating patients with inflammatory bowel disease or uveitis.3
IL-17 inhibitors, such as secukinumab, have been shown to be effective in AS21-23 and can be considered an alternative in radiographic AS if anti-TNF agents fail; however, there are limited data for their use in nr-axSpA. Secukinumab has been shown to be generally well tolerated22 and to have good efficacy in patients suffering from skin psoriasis. Clinicians should be aware that IL-17 inhibitors may exacerbate inflammatory bowel disease.
Targeting other cytokines in the inflammatory pathway, such as IL-23, is also currently being studied.23 Some of the alternative agents with good efficacy in rheumatoid arthritis, such as rituximab and tocilizumab, have unfortunately shown little benefit in AS patients.23 It is encouraging that at least clinicians can now consider IL-17 inhibitors following failure of TNF inhibitors. However, it is recommended that clinicians try an alternative TNF inhibitor first, before commencing treating with IL-17 inhibitors.
Patients with severe deformity may need referral for surgery. Spinal correction procedures and total hip arthroplasty may be needed in patients with refractory pain and structural damage. Anaesthesia in these patients can be a significant undertaking if they suffer from a rigid cervical spine2 and therefore surgical options need careful consideration in terms of risks and benefits to the patient.
CONCLUSION
AS is an autoimmune inflammatory disease that causes severe spinal pain and deformity, and can be associated with extra-articular and systemic features. Untreated, it can result in chronic pain, immobility, and reduced quality of life. Patients complain of inflammatory-sounding back pain and may have other spondyloarthritis features. Investigations can include testing for HLA-B27 and inflammatory markers. Plain radiographs can be normal in early disease; thus, MRI is required to help make a definitive diagnosis. Treatment consists of a multidisciplinary approach and encouraging patients to exercise is essential to maintain mobility. Drug treatment includes anti-inflammatories as first-line and escalation to biologic therapy. Further therapeutic options that target specific IL are currently being developed.