Abstract
Fibromyalgia is a common chronic pain disorder characterised by a robust clinical phenotype with principal features that include widespread pain and tenderness, as well as high levels of sleep disturbance, fatigue, cognitive dysfunction, and emotional distress. Fibromyalgia symptoms occur along a spectrum ranging from mild to severe. The impact on the patient can be very high, with significant effects on personal, recreational, and work activities. The pathophysiology of fibromyalgia is complex and involves abnormal processing of pain and other sensory inputs from the periphery to the brain. In turn, central processes, which modulate this input, are the critical elements that initiate the sequence of events that lead to the clinical phenotype. The functioning of the stress response through its links to pain and other sensory neural processing is a key upstream component of the fibromyalgia cascade. Furthermore, emotional distress appears as a common everyday driver of this process. The mechanisms contributing to the clinical phenotype of fibromyalgia are driven by a top-down process. The aim of this review is to discuss the key central processes that underlie the fibromyalgia clinical phenotype and discuss how these should be the focus of both current management strategies and future research.
INTRODUCTION
Fibromyalgia is a common disorder that affects 3–5% of most studied populations.1 Fibromyalgia is characterised by the presence of widespread muscular and soft tissue pain and tenderness. These symptoms are accompanied by variable levels of poor-quality sleep, fatigue, and cognitive dysfunction, as well as a number of other symptoms that often include headache, abdominal pain, and mood change.2 Generally, these symptoms persist over time but fluctuate in intensity from mild to severe. The resultant burden of disease is high, with a significant impact on personal, recreational, work, and study activities.3-5
While the pathophysiological mechanisms that lie behind the characteristic clinical features are complex, they are now better understood. In this review, the authors examine the evolving understanding of top-down modulatory mechanisms that contribute to the clinical features of fibromyalgia.
DIAGNOSTIC CONSIDERATIONS
Fibromyalgia is diagnosed using a compilation of common and characteristic clinical features. For study purposes, the 1990 American College of Rheumatology (ACR) classification criteria6 have been used for decades. These criteria require the patient to have generalised pain and widespread tenderness, reflecting the altered neurophysiology of the pain-related nervous system that lies behind these critical clinical features.
Over time, enhancements of these criteria have occurred through scoring methodologies; firstly, the number of painful or tender regions (widespread pain index) and, secondly, the degree of unrefreshed sleep, fatigue, and cognitive symptoms, as well as the recent presence of headaches, lower abdominal pain or cramps, and depression (symptom severity scale).7-9 The summation of these two items gives a score ranging from 0–31, which has been given various names, including fibromyalgia research or survey score, polysymptomatic distress scale, or central sensitivity score.10,11 The score allows any individual (i.e., those with and without fibromyalgia) to be placed on a spectrum. The cut-off score for fibromyalgia diagnosis was established as ≥12.9 Patients with ‘sub-diagnostic’ scores may still manifest many features of fibromyalgia; this is termed ‘fibromyalgianess’. As the score increases, it will reflect the presence of more of the clinical manifestations that characterise fibromyalgia. The total score can be used to assess severity or response to treatments.
Two important issues have been identified as a result of the current diagnostic criteria; the first reinforces the links of fibromyalgia to other central sensitivity syndromes. The criteria give most weight to the key presenting feature of fibromyalgia, namely widespread or generalised musculoskeletal pain and tenderness. Additionally, the scores highly rate other clinical features that link predominantly to central mechanisms, including sleep, fatigue, cognition, and mood. These features are common in a number of clinical conditions grouped under the banner of central sensitivity syndromes,12 which are commonly comorbid with fibromyalgia. The sensitivity syndromes include migraine, regional pain syndromes, irritable bowel or bladder syndrome, restless legs syndrome, postural hypotension, and chronic fatigue, among others.
The second issue is the capacity of the criteria to define fibromyalgia as a spectrum disorder. It is important to appreciate that all individuals, both with and without fibromyalgia, will score somewhere along this scale and, therefore, have more or fewer variable musculoskeletal and central symptoms. Thus, variation in severity and fluctuations of key symptoms over time can be documented. The concept of fibromyalgia as a spectrum disorder is very useful in understanding the effect of psychobiological processes, such as stress, on fibromyalgia. Most biological systems are modulated by a number of inputs and give a range of responses along a spectrum rather than being an all-or-nothing response. Fibromyalgia fits with this type of biological response.
CENTRAL SENSITIVITY DIATHESIS AND FIBROMYALGIA
The natural history of fibromyalgia differs between individuals. In many, there is a history of central sensitivity syndrome-related conditions that began during childhood. Headaches, abdominal pain, restless legs, and ‘growing pains’ are not uncommon well before the patient experiences the widespread musculoskeletal symptoms that are required for the diagnosis of fibromyalgia. Thus, it is suggested that there is a central sensitivity diathesis imbedded into the pain-related neural systems of many people. This, in turn, likely relates to a mix of genetic and environmental factors. It is estimated that each factor contributes about 50% to the risk of developing fibromyalgia.13
The trajectory of fibromyalgia symptoms also varies between individuals. In some patients, there is a gradual accumulation of many symptoms over time occurring along the spectrum, captured by the central sensitivity score outlined previously. Eventually, the symptoms will aggregate to such a level that allows diagnosis. As the symptoms increase or decrease, the patient will be rated as worsening, flaring, improving, or going into remission. Other patients report a more rapid onset of symptoms; however, in these cases there is usually previous history of central sensitivity syndrome conditions. In these patients, there is a significant change in the level of any previous pain symptoms or the onset of widespread pain within a short timeframe, measured over weeks or months. Upon the onset of fibromyalgia, the ongoing trajectory will also vary, with some patients continuing to have long-term symptoms while others do not. The symptoms in all patients may fluctuate by varying degrees over the course of time.
NEURAL SENSITIVITY IN FIBROMYALGIA
Patients with fibromyalgia exhibit a number of changes in their pain-related nervous system. In the periphery, there is evidence of neurogenic inflammation, likely contributing to peripheral dysaesthesia and local oedema and pain.14,15 The peripheral neurogenic inflammation is caused by increased secretion of a variety of proinflammatory neuropeptides, cytokines, and chemokines primarily from the unmyelinated C fibres. This antidromic proinflammatory action occurs in fibres that, along with the myelinated Aδ fibres, otherwise transmit information related to actual or potential tissue damage to the pain-related neural pathways. The fibres do this through activation following threshold responses to certain chemical, temperature, or mechanical stimuli, relaying the signal neurons in the outer part of the dorsal horn and, more importantly, to those deep in the spinal cord.
In the spinal cord of patients with fibromyalgia, the neurons deep in the dorsal horn have an increased sensitivity to pain signals transmission;15 this results in enhanced responsiveness to inputs from nociceptors leading to hyperalgesia. More importantly, the sensitisation of deeply placed dorsal horn neurons also changes the sensory response to the input from large fibre mechanoreceptors, in turn causing translation of otherwise innocuous touch and movement input into pain. This process, termed allodynia, is the essential process that causes the generalised pain and tenderness that characterises fibromyalgia. Mechanoreceptor input from deeply placed structures subject to significant biomechanical strains, such as the low cervical and lumbar spines, is likely to contribute to the segmental deep burning pain through the process of referred pain. The sensitisation process in the spinal cord is essential in the development of the widespread pain of fibromyalgia.
The sensitivity of the second order neurons in the spinal cord relates to changes in the function of modulating pathways originating in the brain. For instance, the aberrant function of the descending monoaminergic pathways that involve noradrenaline and serotonin in many patients.16 Improvement of this dysfunction has the potential to decrease peripheral pain and other sensory symptoms in many patients.
There is also an abnormality in the function of brain networks that involve various regions that interact with the midbrain and other centres that relate to these sensory pathways in fibromyalgia.2 These include links between the default-mode network, the pain-inhibitory centres, and other key sensory processing structures, such as the posterior insula.17,18 Modification of the function of these regions has been shown to improve a number of characteristic symptoms of fibromyalgia.19
The Stress Response in Fibromyalgia
There are alterations in the function of the stress response (SR) in fibromyalgia, as manifested by modifications in the hypothalamic–pituitary–adrenal axis and sympathetic function.20,21 The SR refers to the whole-body reaction to a triggering event that might include emotional, chemical, immunological, hormonal, biochemical, or homeostatic components. The SR has two main arms, namely the hypothalamic–pituitary–adrenal arm and the locus coeruleus– noradrenergic system arm. Both arms are activated by various stressors, particularly emotional stressors. Activation may be acute and therefore be part of the fight-or-flight response, or can be subacute and contribute to lesser but more prolonged symptoms. Recurring stimulation of these SR functional units can lead to increased sensitivity of their effector systems, resulting in smaller or alternate new stressors more easily activating the SR.
Thus, emotional distress can activate the traditional SR and lead to the modulation of sensory input to the brain. Each of these components can contribute to symptoms that characterise the clinical phenotype of fibromyalgia. The physiological effects of emotional distress are seen as an initiating factor in this top-down model of fibromyalgia. Emotional distress is generated in the context of a range of psychological factors that influence the processing of life events. Although it is recognised that mind–body interactions are complex and bidirectional, a simple model of this not-fully-understood pathway in regard to fibromyalgia is presented.22
Life events are considered in the context that they occur and may result in thoughts. Under the influence of certain psychological factors, thoughts may be translated into emotions. These modulating factors may include an individual’s personality and their ability to cope with and behavioural response to (e.g., a fear and avoidance response) a new event. For example, in fibromyalgia, symptoms are increased with certain personalities, the ability to cope, the proneness to catastrophise or ruminate, and the tendency to experience anxiety.23 In general, negative thoughts and emotions are associated with fibromyalgia symptoms. The interaction between fibromyalgia, stress, and depression is complex. Depression may be associated with or result from fibromyalgia but is not thought, in the absence of the SR, to be a top-down cause of fibromyalgia.21
Finally, emotions that are generated have physiological effects, and these effects interact with elements of the SR and neural circuits that modulate sensory input to the brain.
Genetic factors also contribute to the risk of developing fibromyalgia.24,25 They generally involve genes that are involved with monoamine and related molecules that are active in stress and pain pathways.26,27 These factors interact with environmental factors to effect modulation of the pain-related neural systems to create a central sensitivity diathesis.
STRESSORS AS TRIGGERS IN FIBROMYALGIA
A trigger is defined as anything that serves as a stimulus and initiates or precipitates a reaction or series of reactions; this includes emotional and biological reactions. In the context of fibromyalgia, a trigger is defined as a specific event that is deemed to exacerbate or cause the onset of symptoms that delineate fibromyalgia. Reported triggers of fibromyalgia have included exposure to certain infectious diseases, chemicals, toxins, physical trauma, and various medical illnesses.2,24 However, the most common trigger is the presence of significant emotional distress.28 This may be present by itself or it may be associated with any of the aforementioned items.
Stressful situations are common throughout life and usually do not trigger any prolonged alteration in neurobiology that might cause longer-term symptoms. However, a range of symptoms may occur at the time of any stressful event. The symptoms will depend on characteristics of the stress, the duration, and the coping skills of the individual.23,28 Eustress indicates a stressor that is perceived by the individual as positive, while distress defines a stressor that exceeds coping strategies and causes negative effects. Emotional distress defines negative stress effects that derive primarily from psychological processes.
PHYSICAL TRIGGERS OF FIBROMYALGIA
A number of physical triggers have been associated with the onset of fibromyalgia. These include infection, physical trauma, and vaccination, among others.29-32 While the methodology, quality, and interpretation of these studies varies, they are in agreement with the clinical observations that there is a strong association between physical trauma and fibromyalgia onset or exacerbation.32 However, the mechanism by which physical trauma triggers the development of fibromyalgia is not clear.33
Peripheral nociceptive input contributes to central sensitisation in animal models. It is also part of the response of the pain-related neural system to acute or persisting peripheral nociception in humans. The resultant increased sensitivity of the relevantly placed spinal cord neurons causes the typical clinical features of secondary hyperalgesia and allodynia, as well as spreading of non-neuroanatomical receptive fields. These peripheral inputs may also contribute to central sensitisation in fibromyalgia.34 It is suggested that there is a continuum of influences from peripheral and central sources contributing to the increased central sensitivity in fibromyalgia.34 For instance, reduction of nociceptive input from symptomatic muscle or joints in patients with fibromyalgia, through active-versus-placebo treatment, caused improvement of background fibromyalgia features, including improvement of pain thresholds.35 However, peripheral influences by themselves seem an unlikely explanation to account for the totality of fibromyalgia symptoms.16 Fibromyalgia is more than amplification of responses in the pain- related nervous system. It involves increased response to a variety of other sensory inputs, such as light and noise, as well as background sleep, fatigue, cognition, and mood changes. These all relate to activities of the central nervous system.
Hence, to ascribe physical trauma as the key pathophysiological process causing fibromyalgia is too simplistic. Each of the studied and clinically observed cases of physical trauma have significant associated psychological aspects, often not considered in the term ‘physical’ trauma. Psychological response is inherent in any situation of physical trauma and, in the context of fibromyalgia, is deemed to be the major consideration for causation.
PSYCHOLOGICAL TRIGGERS AND FIBROMYALGIA
Defined Stressors
While there are no high-quality prospective studies that examine psychological stressors in fibromyalgia, there are a lot of cross-sectional and case control studies that indicate a strong association between emotional distress and fibromyalgia.32
Much attention has been given to psychological stressors that can be relatively easily identified. One common stressor is exposure to sexual or physical abuse.36-52 These studies are subject to retrospective bias but generally indicate increased rates of fibromyalgia compared to various control groups in those exposed to this type of psychological trauma. A systematic review and meta-analysis of studies in this area concluded that there is an increased risk of fibromyalgia after psychological-related trauma.53,54
Post-traumatic stress disorder (PTSD) represents another well-defined situation in which emotional distress dominates the clinical picture.32 There are high rates of PTSD in patients with fibromyalgia55-58 and high rates of fibromyalgia in those with PTSD.59-64 In a study of 395 patients with fibromyalgia, 45.3% had PTSD and 66.5% developed fibromyalgia after the onset of PTSD.65 While both PTSD and fibromyalgia are defined for study purposes using strict validated criteria, both disorders exist on a spectrum. Further exploration of the associations between components of each disorder may show even stronger links between this type of emotional distress and fibromyalgia.
Background Stressors
Background psychological stressors are common and often not easily labelled as ‘post-traumatic’ because they are part of everyday life events. The development of emotional distress in response to these stressors and activation of the SR varies significantly in different individuals.
While genetic factors influence function at numerous levels in stress-related brain and spinal pathways, the majority of other contributing factors relate to psychological mechanisms. As indicated earlier, the response to everyday life events may trigger emotional distress in individuals when certain psychological buffers fail to work effectively. This situation may arise if the individual is prone to negative thoughts, or if there is excessive worry or rumination. Those who catastrophise easily or who lack control over an event are prone to develop emotional distress and activate the SR.
The responses of individuals to life stressors occur on a spectrum. Some only develop emotional distress with extreme provocation, while others will activate the SR with minimal stimuli. Most are somewhere in between these two extremes, with variable levels of emotional distress. This is akin to the symptoms of fibromyalgia, for which fluctuations in symptoms is characteristic. These two processes are linked through the process of central sensitisation; the SR is the initiator and the fibromyalgia clinical phenotype is the output. These processes are summarised in Figure 1.
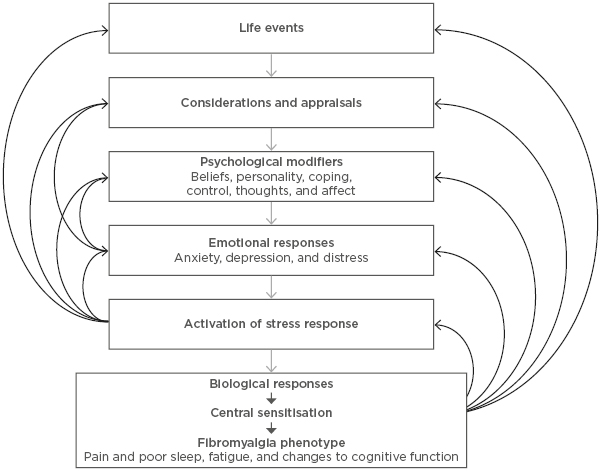
Figure 1: Key elements contributing to the top-down model of fibromyalgia.
Life events, such as psychological triggers, are appraised and translated into emotions under the influence of various psychobiological factors. Emotional distress activates biological responses, which include central sensitisation, the basis of fibromyalgia symptoms. Many feedback loops influence the phenotypic outcome.
IMPLICATIONS FOR MANAGEMENT OF FIBROMYALGIA
The top-down model allows for a better understanding of fibromyalgia management strategies. Strategies that focus on stress reduction, such as those that include relaxation, form the basis of management. These strategies are enhanced by those that are activity-based. Exercise and strength training benefit both physical and mental health and may have potent effects on fibromyalgia symptoms. Drugs that target sleep, stress, or the sensitisation processes are associated with better outcomes in fibromyalgia.66 These so-called pain-modulators target the sensitisation mechanism of fibromyalgia and are contrasted to the action of potent analgesics, such as pure opioid analgesics, that are not effective in fibromyalgia.66
Future therapies that better modulate the process of central sensitisation will be dependent on increased understanding of the effects of the SR on neural sensitivity, particularly those involved in pain perception. Evidence-based strategies that modulate this response will likely range from those that are psychologically based to those that are pharmacologically based and may even include novel treatments such as transcranial magnetic stimulation.
CONCLUSION
Fibromyalgia results from a cascade of neurophysiological responses that are primarily driven by brain-related mechanisms. Among these are links between thoughts, emotional responses, the SR, and sensory processing. Further research and management strategies for this common, high-impact clinical disorder need to start at the top.