Abstract
Primary Sjögren’s syndrome (pSS) is a relatively common disease and one of the most common rheumatic diseases of autoimmune and inflammatory origin. It is primarily associated with symptoms of dryness, mainly in the mouth and eyes, but it can also manifest in the internal organs. Epidemiological studies have highlighted that elderly-onset pSS (EOpSS) is common, and it is known that sicca syndrome is a feature often observed in the elderly and can be induced by several factors. However, the presence of autoantibodies in older patients with sicca syndrome can be age-related and does not mean pSS is present. This review article presents the most important elements for making a correct diagnosis of EOpSS and considers clinical and/or laboratory differences between older and younger pSS patients. According to data from the literature, EOpSS is not a distinct subset of disease when compared with younger-onset pSS.
INTRODUCTION
Primary Sjögren’s syndrome (pSS) is an autoimmune disease that typically has exocrine gland involvement and can lead to sicca syndrome, although internal organs may also be affected. Due to the ever-growing access to rheumatological diagnostic assessments, including immunological (presence of autoantibodies) and histopathological (salivary gland biopsy), early diagnosis is possible. In the elderly, sicca syndrome is a common feature that can be induced by several factors. In addition, the presence of autoantibodies in older patients with sicca syndrome can be age-related and does not mean pSS is present. Knowledge of all clinical elements is very important for a correct diagnosis of elderly-onset pSS (EOpSS). The aim of this review article is to provide information to facilitate an understanding of how to correctly diagnose EOpSS; furthermore, the clinical and/or laboratory differences between EOpSS and younger-onset pSS are also highlighted.
PRIMARY SJÖGREN’S SYNDROME PATHOGENESIS
The pathogenesis of pSS is characterised by epithelial damage, release of autoantigens, and activation of innate and acquired immunity; however, B lymphocytes and the production of autoantibodies play the main role in pSS pathogenesis.1,2 The factors that trigger the pathophysiological phenomena in pSS include genetic predisposition (for genes encoding human leukocyte antigen [HLA]-B8, HLA-Dw3, HLA-DR3, and HLA-DRw52), infection (especially Epstein–Barr virus),3 environmental factors, such as ultraviolet radiation,4 and hormonal disorders.5,6 Smoking has also been associated with pSS development; however, recent observations have been made about the lack of influence, and even positive effect, of smoking on the course of pSS.7,8 Among antibodies against extractable nuclear antigens, those against small ribonucleoproteins SS-A (Ro) and SS-B (La) are particularly important in pSS diagnosis and pathogenesis.9
EPIDEMIOLOGY OF PRIMARY SJÖGREN’S SYNDROME
Epidemiological data show that pSS is a relatively common disease and one of the most common rheumatic diseases of autoimmune and inflammatory origin; however, the incidence and prevalence may vary depending on the diagnostic criteria used.10 The prevalence of pSS, based on various sources, ranges from 0.72–2.70% of the general population, but some studies have reported prevalence rates as high as 5.00%.11 Many studies have confirmed that pSS is more common in women (female: male ratio of 9:1) and mainly affects individuals between the ages of 40 and 60 years, with the disease most frequently occurring in people around 50 years of age.10-12
EPIDEMIOLOGICAL DATA FOR ELDERLY-ONSET PRIMARY SJÖGREN’S SYNDROME
EOpSS has an estimated overall prevalence of approximately 3% but epidemiological data are very heterogeneous and prevalence depends on variables such as geographic area and, above all, diagnostic criteria. Relatively old data presented in a study by Drosos et al.,13 in which 62 healthy volunteers with a mean age of 81 years (range: 67–95 years) were examined, confirmed pSS in 4.83% of the study group. The authors suggested that pSS in elderly people is subclinical, benign, and relatively common.13 In another study conducted in 2008,14 researchers showed that in an elderly group (aged 71–74 years) pSS was confirmed in 3.39% (95% confidence interval [CI]: 2.77–4.14) according to the European classification criteria from 1993, and in 1.40% (95% CI: 1.02–1.92) according to the revised European classification criteria from 1996. The prevalence of pSS in the younger group (aged 40–44 years) was lower, totalling 0.44% (95% CI: 0.34–0.57) and 0.22% (95% CI: 0.15–0.32), respectively.14 In an Italian cohort,15 6% of patients had EOpSS, while in a Tunisian cohort this value was higher at 30%.16 Johansson et al.17 highlighted that symptoms of dryness were reported in >30% of elderly people, with the percentage increasing with age, and that symptoms were more frequently expressed in women. In the Lin et al.18 population-based study, dry eye symptoms had higher prevalence in elderly Asian populations than in Caucasian populations, with as much as 47.5% of the Asian group diagnosed with dry eye and in need of topical treatment.
CLINICAL FEATURES OF PRIMARY SJÖGREN’S SYNDROME
Destruction of the lacrimal or salivary glands is the cause of the most common complaint associated with pSS; however, such dryness can also affect the mucous membranes of the bronchial tree, gastrointestinal tract, and vagina, with the most commonly occurring symptoms being bronchitis and coughing. Some more general symptoms have been reported by pSS patients, and some of the particularly frequent symptoms include fatigue, general weakness, and chronic pain; these may also be a cause of diagnostic confusion, especially in older patients.
The common organ lesions observed in pSS are presented in Table 1. Of all the phenomena listed, changes in the lungs deserve particular attention because of their frequency; for example, interstitial lung disease occurs in approximately 10–20% of pSS cases.19 Most often this is a nonspecific interstitial pneumonia, but these changes may remain unrecognised for a long time since the classic radiological examination is insufficient to establish a diagnosis at an earlier stage.20 In older patients, the higher likelihood of infection and drug resistance can represent specific critical issues.
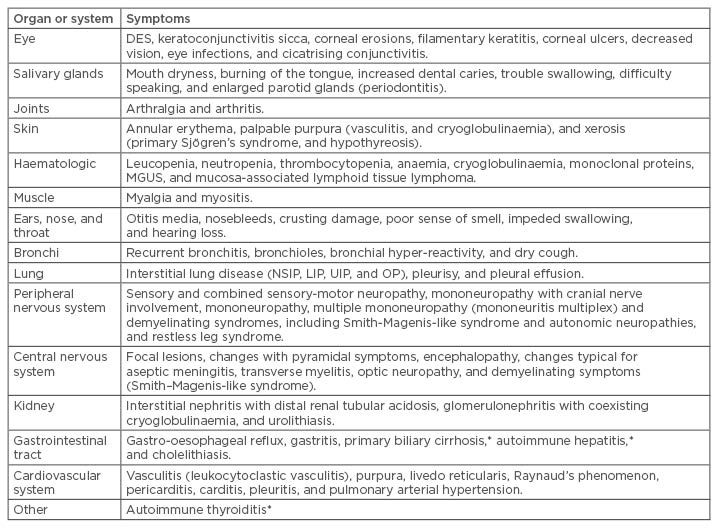
Table 1: Organ and system-specific symptoms of primary Sjögren’s syndrome.
*Autoimmune diseases commonly accompanying primary Sjögren’s syndrome.
DES: dry eye syndrome; LIP: lymphocytic interstitial pneumonia; MGUS: monoclonal gammopathy of undetermined significance; NSIP: nonspecific interstitial pneumonia; OP: organising pneumonia; UIP: usual interstitial pneumonia.
CLASSIFICATION AND DIAGNOSTIC CRITERIA
Criteria for the diagnosis of pSS have evolved over the years since the discovery of the disease, along with the broadening of immunological knowledge and the improvement of assessment techniques for the symptoms of dryness. All principal cohorts in the literature are based on the classification criteria of the 1993 European Community Study Group (ECSG)21 and the 2002 American European Consensus Group (AECG) criteria.22 The AECG criteria built upon previous preliminary criteria proposed in 1993 by a European collaborative group. It considered six items, two of which were subjective (ocular and oral symptom complaints by the patients) and four based on objective findings. These objective findings are Schirmer’s test, Rose Bengal score according to the van Bijsterveld score, minor salivary biopsy with a focus score (FS) >1, and objective evidence of salivary gland involvement defined by a positive result for at least one of the following diagnostic tests: unstimulated whole salivary flow <1.5 mL in 15 minutes; parotid sialography showing the presence of diffuse sialectasis (punctate, cavitary, or destructive pattern), without evidence of obstruction in the major ducts; salivary scintigraphy showing delayed uptake, reduced concentration, and/or delayed excretion of trace; and presence in the serum of antibodies to Ro (SSA) or La (SSB) antigens, or both. In patients without any potentially associated disease, pSS may be defined as the presence of four of the aforementioned six items (histopathology and autoantibodies are mandatory) or presence of three of the four objective criteria.
At the end of 2016, the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) presented jointly established pSS diagnostic criteria.23 Of all the laboratory tests, diagnostic importance was attributed only to SSA/Ro autoantibodies (3 points). Ocular staining score and Schirmer’s test were retained as part of the ophthalmologic examination (1 point), while the measurement of unstimulated salivary flow was proposed for the assessment of salivary gland function (1 point). Histopathological examination of minor salivary gland biopsies with the assessment of the FS of the infiltrate cells remained an important element of diagnosis (FS >1 gives 3 points). The diagnosis is made if a patient presents with a sum of ≥4 points, but a cut-off of 5 points instead of 4 raises the specificity of the criteria from 89% to 98%.24
For the diagnosis of pSS, it is important to assess the established exclusion criteria, such as head and neck radiation treatment, active hepatitis C virus (HCV) infection (confirmed using PCR), AIDS, sarcoidosis, amyloidosis, graft versus host disease, and immunoglobulin G4-related disease. Additionally, in the evaluation of dry eye symptoms, patients using eye drops for glaucoma daily and those who have had corneal surgery or cosmetic eyelid surgery in the last 5 years are scored 0 points.
Furthermore, it should be considered that some drugs, even when not applied locally in the form of eye drops but administered in other ways, reduce the secretion of tears or saliva.25 These drugs include anticholinergics, antidepressants (tricyclic or selective serotonin reuptake inhibitors), antihypertensives (terazosin, prazosin, clonidine, and atenolol, antihistamines, antireflux drugs, diuretics, and benzodiazepines.
Biopsy of minor salivary glands remains a gold standard for proving inflammation and infiltration of the salivary glands by mononuclear cells. This assessment should be performed by a pathologist with expertise in the diagnosis of focal lymphocytic sialadenitis and FS count.24 FS means no less than 50 mononuclear cells per 4 mm2 of the glandular section.
SYMPTOMS AND DIAGNOSTIC CRITERIA IN OLDER PATIENTS
The relationship between disease, drugs, radiotherapy (RT), age-related glandular functions, and sicca syndrome is widely described in the literature. In the case of EOpSS, these factors should be taken into account to avoid misdiagnosis. For lachrymal and salivary glands, for example, older age is associated with a reduction of tear and/or saliva production. Since the likelihood of a positive Schirmer’s test result gradually increases with advancing age,26 the test in an elderly individual cannot be used in isolation to diagnose EOpSS. Furthermore, in clinical practice, older persons have comorbidities and are often prescribed numerous drugs that can induce salivary and/or lachrymal gland dysfunction or alter laboratory test results. Indeed, it is understandable that drugs with antagonistic actions on autonomic receptors and that are used to treat dysfunction in the various effectors of the autonomic nervous system may also affect the functions of salivary glands and thus cause oral dryness. Some patients taking enalapril and lisinopril present with xerostomia,25,27 which can also be induced by nonsteroidal anti-inflammatory drugs, such as ibuprofen, naproxen, and piroxicam.27 Xerostomia is also frequent in patients with head and neck cancer being treated with RT. Usually, radiation-induced xerostomia has an early onset; in the first week after therapy, half of patients present with a decrease in salivary flow and, after head and neck RT, salivary glands have a limited capacity for repair, especially with mean doses above 40 Gy.28
HCV infection represents another exclusion criterion. In 1992, Haddad et al.29 reported lymphocytic sialadenitis in 57% of HCV-infected patients and 5% of controls. Clinical pathology and biologic similarities between these two diseases suggest common pathogenic pathways.29 In clinical practice, the availability of new, very effective drugs for HCV eradication underlines the necessity to reconsider the diagnosis of pSS once the absence of serum HCV RNA has been obtained.
In older persons, a biopsy of the minor salivary glands can constitute an important diagnostic conundrum. In labial salivary gland (LSG) biopsies, a focal lymphocytic sialadenitis with >50 mononuclear cells in a periductal or perivascular localisation is considered the most specific finding for pSS diagnosis. A protocol published in 2011 by the Sjögren’s International Clinical Collaborative Alliance (SICCA) underlined that these foci must occur adjacent to normal appearing acini.30 In older patients, acinar atrophy and fibrosis can be age-related or due to expression of nonspecific chronic sialadenitis, which can introduce a confounding element (for example, availability of <4–6 glands suitable for diagnostic evaluation). More recently, the Sjögren’s histopathology workshop by the EULAR Sjogren’s Syndrome Experimental and Translational Investigative Alliance Study Group (ESSENTIAL) provided standardised consensus guidance for the use of LSG histopathology in the classification of pSS. The diagnostic importance of foci that are adjacent to normal parenchyma was emphasised. Furthermore, recommendation number 6 was that the extent of the atrophic features should be graded (as mild, moderate, and severe) in addition to the presence or absence of focal lymphocytic sialadenitis; this had a level C strength of recommendation. Another recommendation (number 10) concerning the necessity that all foci should be included in the FS and in foci calculations, even when adjacent to abnormal acinus or ducts, obtained a level D in guidance proposed for the clinical trials.31
AUTOANTIBODIES AND AUTOIMMUNE DISEASES IN THE ELDERLY: THE IMPORTANCE OF IMMUNOSENESCENCE
In elderly individuals, some aspects of the immune system function are lost. During an individual’s lifetime, the immune system undergoes changes: the mechanisms of acquired immunity may, over time, outweigh the pool of naïve lymphocytes that have not yet come into contact with an antigen and, as such, the number of antibodies and immunological complexes grows, as well as the number of memory cells due to the numerous pathogens encountered.32 In the elderly, an increase in proinflammatory cytokines (age-associated low-grade inflammation) is also observed.33 With age, the frequency of antinuclear antibodies (ANA) and other antibodies increases, but the incidence of autoimmune diseases is less frequent in individuals >75 years than in the 30–50 year age range. Among the systemic diseases of connective tissue, systemic lupus erythematosus occurs in elderly individuals less often than pSS.34 The antibodies most frequently present in pSS are antibodies against the small ribonucleoproteins SSA/Ro and SSB/La. Researchers have shown that patients diagnosed before 45 years of age have higher anti-SSA and SSB autoantibody concentrations (62.5%) than patients with EOpSS (20.8%).35,36 The presence of rheumatoid factor (RF) is often observed in healthy elderly people, possibly as a consequence of the age-related immune deregulation.37 In the above cited papers,35,36 RF concentrations in patients with pSS were higher in the group with earlier diagnosis before the age of 45 years.
PRIMARY SJÖGREN’S SYNDROME AND CANCER RISK
Patients with pSS are particularly vulnerable to the development of lymphomas, including non-Hodgkin’s lymphoma from B lymphocytes, although less frequently from T and natural killer cells. Patients with pSS present 9–44-times more often with lymphomas than healthy populations.38,39 Marginal zone B cell lymphoma is most often described, which includes mucosa-associated lymphoid tissue lymphoma. Less frequently, but with great clinical relevance, disseminated lymphoma from large B lymphocytes occurs (diffuse large B cell lymphoma).
ELDERLY-ONSET PRIMARY SJÖGREN’S SYNDROME AND CANCER RISK
Age itself is one of the most important elements for the development of malignancies. Despite this, data regarding the neoplastic status of EOpSS patients are very scarce and primarily highlighted by case reports.40 One study evaluated the incidence and standardised incidence ratio of breast cancer in a cohort of elderly patients with some chronic autoimmune diseases and did not find an increased risk in the group of patients with EOpSS.41
DIFFERENCES BETWEEN YOUNGER AND OLDER PATIENTS WITH PRIMARY SJÖGREN’S SYNDROME
As shown in Box 1, some differences can be underlined in EOpSS compared to younger pSS patients.
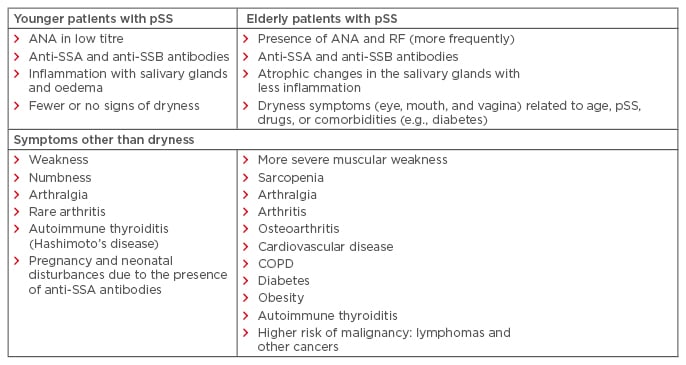
Box 1: Differences in primary Sjögren’s syndrome between a group of older and younger patients.
ANA: antinuclear antibodies; COPD: chronic obstructive pulmonary disease; pSS: primary Sjögren’s syndrome; RF: rheumatoid factor; SSA: serum of antibodies to Ro; SSB: serum of antibodies to La.
COHORT STUDIES PRESENT IN THE LITERATURE
In a search of PubMed, four studies were identified that reported on cohorts of patients with EOpSS, totalling 99 patients (Table 2). In these studies, disease onset was determined based on the occurrence of symptoms strongly suggestive of pSS; in three of the studies, elderly onset was set at age 65 years, but in the García-Carrasco et al.42 study, it was set at 70 years. Each study used the diagnostic criteria commonly being used at the time of publication.
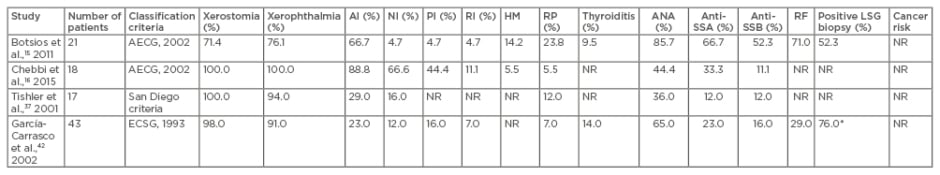
Table 2: Clinical manifestations, laboratory data, classification criteria, histological results, and cancer risk in four literature studies.
*LSG biopsy was performed in 25 of the 43 patients.
AECG: American European Consensus Group; AI: articular involvement; ANA: antinuclear antibodies; ECSG: European Community Study Group; HM: haematological manifestations; LSG: labial salivary gland; NI: neurological involvement; NR: not recorded; PI: pulmonary involvement; RF: rheumatoid factor; RI: renal involvement; RP: Raynaud’s phenomena; SSA: serum of antibodies to Ro; SSB: serum of antibodies to La.
A high percentage of patients with ANA positivity presented in the Botsios et al.15 cohort; this was only partially confirmed in the other three studies. Up to 20% of healthy people can have ANA positivity, and this probability is much higher in older people as a consequence of the generally accepted hypothesis that immunosenescence causes a decrease of self-regulatory mechanisms with increased autoantibody production. With the exception of the Chebbi et al.16 data, the presence of anti-SSA and anti-SSB were similar in all studies. Given the absence of phenotypic features associated with isolated anti-SSB autoantibodies, and the low negative and positive likelihood ratios for the diagnosis of pSS, the recent classification of the ACR and EULAR23 excluded anti-SSB-positivity from the diagnostic criteria. Differences between studies were found in clinical characteristics of patients, with neurological and pulmonary involvement more often observed in the Chebbi et al.16 cohort and Raynaud’s phenomenon more frequently observed in the Botsios et al.15 cohort.No data regarding cancer risk were present in the four studies. LSG biopsies were performed in two cohorts.15,42
Lastly, when the authors compared older-group data with younger-group data, differences were only highlighted in the study by Chebbi et al.16 In this study, pulmonary involvement was more frequent in the older group (although not statistically significant), whereas the difference in levels of ANA, anti-SSA, and anti-SSB was statistically significant in the younger group. In the other three studies, clinical and laboratory results of EOpSS patients were quite similar to those in younger patients. Demographic factors and differences in genetic predisposition have a potential role in explaining these differences. The reduced expression of immunological features in patients with EOpSS (more evident in the Chebbi et al.16 cohort but present in all the cohorts considered) may reflect the senescence of the immune system.
CONCLUSION
According to the data found in the literature, it can be concluded that EOpSS is not a distinct subset of disease, unlike elderly-onset rheumatoid arthritis or elderly-onset systemic lupus erythematosus, for example. However, age-related manifestations, such as dryness symptoms, ANA, and RF positivity, as well as all the comorbidities and therapies that can induce sicca syndrome, should be carefully evaluated to avoid misdiagnosis.
Similarly, Schirmer’s test, due to age-related glandular involution, may be less useful than ocular staining score as a confirmation test for keratoconjunctivitis sicca in EOpSS patients. LSG biopsy is performed less frequently in older patients, and there is a need to achieve a consensus among experts on how to differentiate pSS lesions from the age-related degenerative and atrophic lesions of salivary glands. Futhermore, the relationship between EOpSS and cancer risk must be evaluated through further studies on ad hoc cohorts, taking into account that age is one of the most important risk factors for the development of malignancies. Lastly, the specificity and sensitivity of AECG criteria should be evaluated in a comparative way between older and younger age groups; currently, this evaluation is scarcely considered. Additionally, no data are available on EOpSS regarding the specificity and sensitivity of the criteria proposed in 2016 by the ACR/EULAR collaborative group.








