Abstract
COVID-19 has variable clinical presentations, severity, and multisystem involvement. Some patients have encountered arthralgia and myalgia symptoms. Reactive arthritis is an emerging musculoskeletal manifestation post-COVID-19 infection. The authors report a 29-year-old male, previously healthy, who developed acute left hip arthritis 8 weeks after recovering from severe COVID-19 pneumonia. It was associated with bilateral avascular necrosis of femoral heads, with medullary bone infarctions at both proximal tibias. Human leukocyte antigen-B27 was positive and synovial fluid culture, autoimmune workup, crystals, and infectious aetiologies were negative. The patient’s clinical condition improved with the use of non-steroidal anti-inflammatory drugs and physiotherapy. To the best of the authors’ knowledge, this is the first case reported of hip arthritis and avascular necrosis following the COVID-19 infection. In addition, the authors reviewed the literature for the reported reactive arthritis cases post-COVID-19 infection and management outcomes.
INTRODUCTION
The incidence of the COVID-19 infection is increasing globally, with an estimated mortality rate of 3.5%. The cardinal presenting symptoms of COVID-19 infection are fever, cough, and shortness of breath. Gastrointestinal symptoms, and musculoskeletal complaints (arthralgia and myalgia) have been encountered in 15–30% of patients.1 The clinical severity ranged from mild disease to severe pneumonia, multi-organ failure, and death. Cytokine storm or cytokine release syndrome might contribute to extra-pulmonary involvement of COVID-19 such as acute kidney injury, venous thrombosis, neurological complications, and hepatic and myocardial injury.2 Reactive arthritis is an emerging musculoskeletal (MSK) manifestation post-COVID-19 infection. The authors report a case of acute arthritis in the left hip and bilateral avascular necrosis (AVN) of femoral heads, with medullary bone infarctions at both proximal tibias developed 8 weeks after severe COVID-19 pneumonia.
CASE DESCRIPTION
A 29-year-old athletic male from Jordan, who was previously healthy, tested positive for COVID-19 on 20th May 2020, with initial presenting symptoms of fever, myalgia, breathing difficulty, and a cough. They were a non-smoker and did not drink alcohol. There was no previous allergy and no family history of rheumatologic diseases.
The patient’s clinical condition deteriorated with development of acute respiratory failure due to respiratory distress syndrome and severe COVID-19 pneumonia. They required mechanical ventilation and prolonged critical care in another hospital. They were treated with combination of experimental anti-COVID-19 therapies, including favipiravir (1,600 mg orally twice daily [BID] on Day 1, and 600mg orally BID for 10 days), hydroxychloroquine (400 mg orally BID on Day 1, then 200 mg orally BID for 4 days), tocilizumab (400 mg intravenously [IV] once), and methylprednisolone (40 mg given IV daily for 5 days). They had nosocomial infection (urosepsis) and were treated successfully with broad-spectrum antibiotics (ertapenem). The patient required physiotherapy for critical illness neuropathy and chest physiotherapy, with gradual improvement. They were discharged on 3rd August 2020 in a stable clinical condition with mild left hip pain.
On 3rd November 2020, the patient presented to the authors’ hospital with a 2-day history of fever, shortness of breath, worsening productive cough, and severe left hip pain, with limited mobility. The patient noticed mild non-traumatic left hip pain after their first hospital discharge, which got worse over time. A review of systems was negative for back pain, other joint involvement, skin rash, oral or genital ulcers, diarrhoea, and eye redness. They had no recent contact with sick individuals or recent travel. In the emergency department, they were febrile (38.1 °C), had sinus tachycardia (116 beats/min), and were in mild respiratory distress (respiratory rate of 25 breaths/min). Their blood pressure was 115/75 mmHg and BMI was 22.02 kg/m2. There was evidence of bilateral crackles involving the upper and middle zones of the lungs. Their cardiovascular exam was normal. A MSK examination revealed tenderness in left hip area, with limited range of movements. Other physical examinations were unremarkable.
Their laboratory investigations revealed normal liver, kidney, and thyroid function tests, and for uric acid. Inflammatory markers were elevated, including C-reactive protein (66.000 mg/L), erythrocyte sedimentation rate (102.000 mm/hour), and D-dimer (2.680 mg/L) levels (Table 1). Deep venous thrombosis and pulmonary embolism were ruled out by an ultrasound Doppler of lower legs and a CT pulmonary angiography, respectively. They had evidence of post-COVID-19 pulmonary changes, including peribronchial consolidation in the upper lobes and traction bronchiectasis (Figure 1A and B). They were admitted as case of acute pneumonia and was started on oral amoxicillin-clavulanic acid (1 g every 8 hours for 5 days) and albuterol/ipratropium via nebuliser.
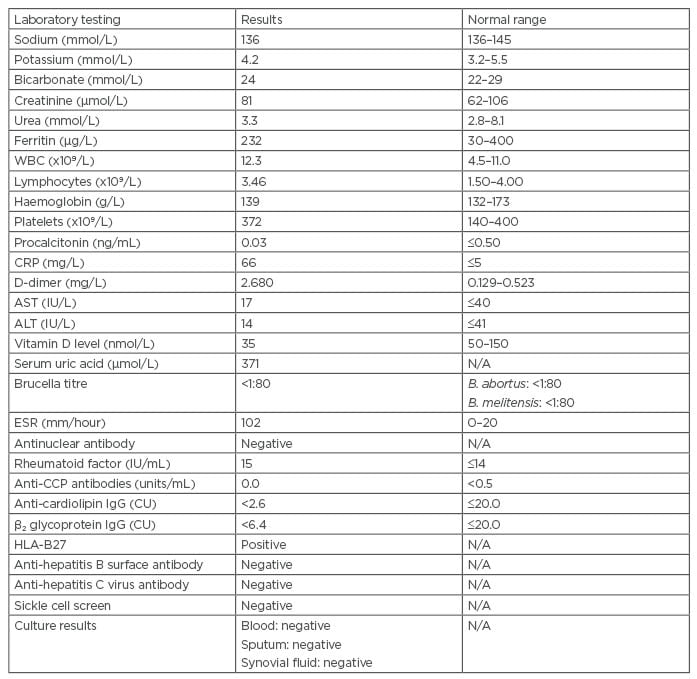
Table 1: Laboratory results of the authors’ patient.
ALT: alanine aminotransferase; AST: aspartate aminotransferase; B. abortus: Brucella abortus; B. melitensis: Brucella melitensis; CRP: C-reactive protein; CU: chemiluminescent units; CCP: cyclic citrullinated peptide; ESR: erythrocyte sedimentation rate; HLA: human leukocyte antigen; N/A: not applicable; WBC: white blood cell.
Baseline imaging tests for the left hip were not taken previously. The left hip X-rays were normal. MRI with gadolinium of the hip showed left hip joint effusion, with peripheral synovial enhancement, AVN of subtrochanteric femoral heads, and medullary bone infarctions located in distal right femur and both proximal tibias (Figure 1C and D).
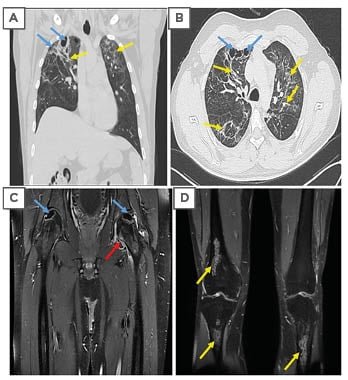
Figure 1: CT and MRI scans of the patient.
A) and B) Chest CT after 2 months following severe COVID-19 pneumonia showing parenchymal distortion, fibrotic changes (yellow arrows), and multiple subpleural cysts (blue arrows). C) and D) MRI of the bilateral femurs (coronal T1 fat-saturated post-contrast images), geographic abnormal signals involving bilateral superolateral femoral heads (blue arrows); left hip joint effusion (red arrow), with peripheral synovial enhancement; and medullary serpiginous abnormal signals in the distal right femur and bilateral proximal tibias in keeping with multi-focal medullary bone infarctions (yellow arrows).
Autoimmune workup was negative as well as infectious tests (including HIV, hepatitis B and C virus, Brucella, mycoplasma, and tuberculosis [hl]Table 1[/hl]). Blood and sputum cultures were negative. On Day 6 of admission (after 1 day of completing the antibiotic course), the patient’s left hip pain did not improve, and they underwent ultrasound guided aspiration of the left hip effusion. Fluid analysis revealed turbid, yellow fluid, red blood cell count of 18,000 cells/mm3, white blood cell count of 45,684 cells/mm3, 91% neutrophils, no crystals, and the culture was negative. Orthopaedic and rheumatology teams were consulted, and the patient was treated with anti-inflammatory medications (40 mg of parecoxib given IV for 4 days, then naproxen 500 mg BID) for reactive left hip arthritis and AVN.
X-rays of the lumbosacral spine and sacroiliac joints were normal. On Day 8 of admission, they underwent left femoral head core decompression by the orthopaedic team. In addition to regular physiotherapy, their clinical condition improved dramatically within 2 weeks of hospitalisation period. They were discharged home on naproxen 500 mg BID and an albuterol inhaler. The human leukocyte antigen (HLA)-B27 came back positive after discharge on Day 14. After a 2-week follow up at telemedicine clinic, the patient reported no pain at left hip joint and good mobility with use of naproxen. At the rheumatology clinic follow-up after 6 weeks, the patient was in a stable clinical condition and non-steroidal anti-inflammatory drug (NSAID) therapy was stopped.
DISCUSSION
Reactive arthritis is a form of spondyloarthropathy, with reported incidence of 0.6–27.0 per 100,000 in population-based studies.3 It affects mostly young males (aged 20–50 years) and occurs 1–6 weeks following symptoms of a gastrointestinal or genitourinary (GU) infection. About 50% of cases are associated with HLA-B27.4 The underlying pathophysiologic process is not well established and linked to an immune-mediated activated cytotoxic-T cells following an infection, inflammatory cytokines production, synovial injury, and molecular mimicry.5,6
Reactive arthritis manifests as asymmetrical oligoarthritis, mainly involving peripheral or axial joints of lower extremities. Extra-articular manifestations associated with reactive arthritis include MSK (enthesitis, tendinitis, dactylitis), eye (conjunctivitis, episcleritis, anterior uveitis), GU (urethritis, cervicitis, cystitis, prostatitis, circinate balanitis), mucosal and skin involvement (mucosal ulcers, keratoderma blennorrhagica, erythema nodosum), nail changes, and, rarely, cardiac involvement (conduction, aortic valve abnormalities).6,7 The systematic approach and screening for multisystem involvement is essential for the diagnosis of reactive arthritis.
The most commonly reported infections associated with reactive arthritis are HIV, bacterial GU infections (Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma hominis, and Ureaplasma urealyticum), and gastrointestinal infections (Salmonella, Shigella, Campylobacter jejuni, Yersinia enterocolitica, and Clostridium difficile). Chlamydia pneumoniae can be associated with acute respiratory infection and reactive arthritis.6,8 Screening for relevant infectious aetiologies in reactive arthritis is required. Inflammatory mediators might be elevated with negative culture results. Testing for HLA-B27 and other autoimmune work-up is needed. In the acute phase, plain radiographs might be normal or reveal non-specific joint findings. MRI or ultrasonography are more sensitive in detecting peripheral synovitis, enthesitis, and sacroiliitis.4,6,9
On the other hand, COVID-19 associated reactive arthritis is rarely reported. The authors had a review of 11 cases of post-COVID-19 reactive arthritis.10 The authors conducted a literature review with a search from January 2020 to August 2021 and using PubMed, Google Scholar, and Mendeley. Search keywords were appropriate to the topic and not limited to: “reactive arthritis,” “joint pain,” “COVID-19,” “SARS-CoV-2 infection,” “effusion,” and “musculoskeletal.” A total of 25 cases fulfilled the inclusion criteria. The mean age of patients was 42.36 years and 56% of patients were males (Table 2).4,9,11-31 The median duration of reactive arthritis diagnosis from COVID-19 infection ranged from 4 days to 8 weeks. Around half of the patients had mild COVID-19 infection (n=14 [56%]), while 16% of patients had either moderate or severe disease. Interestingly, there was similar range of joints involvement reported in COVID-19 reactive arthritis, including monoarticular involvement (36%), oligoarticular (2–4 joints; 32%), and polyarticular involvement (>4 joints; 32%). Seven patients had synovial fluid analysis reported as inflammatory with negative culture, crystals, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) PCR result. The authors’ patient had delayed left hip arthritis, occurring 8 weeks after COVID-19 infection.
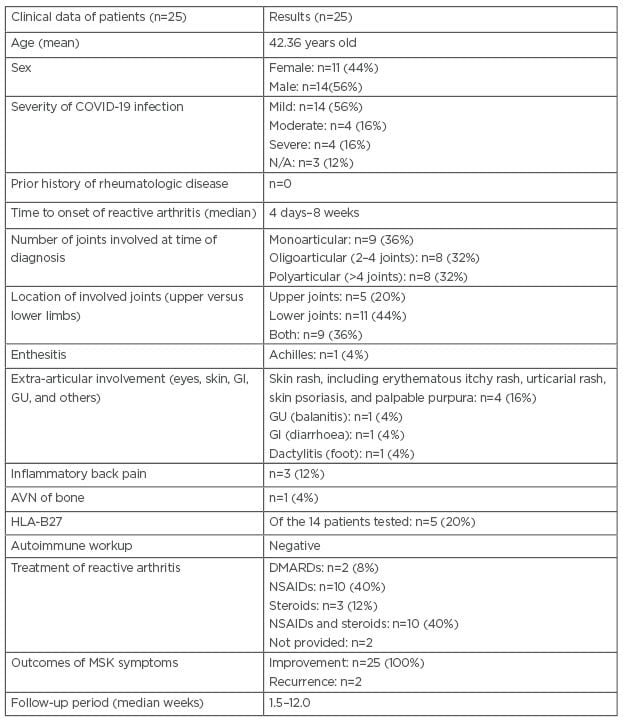
Table 2: Characteristics of the 25 reported cases (including the authors’ patient) of reactive arthritis post-COVID-19 infection.
AVN: avascular necrosis; DMARD: disease-modifying anti-rheumatic drug; GI: gastrointestinal; GU: genitourinary; HLA: human leukocyte antigen; MSK: musculoskeletal; N/A: not applicable; NSAID: non-steroidal anti-inflammatory drug.
Extra-articular manifestations were identified in 40% of patients, including skin rash (erythematous itchy rash, urticarial rash, skin psoriasis, palpable purpura), diarrhoea, dactylitis, wrist tendinitis, Achilles enthesitis/tendonitis, and balanitis. Of all the patients, 12% had inflammatory back pain. Autoimmune workups were unremarkable in most of cases. HLA-B27 testing was completed in 14 patients and only five patients (20%) had positive results. Plain radiographs and MRI were used to identify extent reactive arthritis, with findings ranging from tenosynovitis, joint effusion, and sacroiliitis being noted in some patients who had tested positive for HLA-B27.
In this cohort, 40% of patients were managed with either NSAIDs alone, or a combination of NSAIDs and steroid (oral, intra-articular). Other patients were managed with corticosteroids (n=3 [12%]), while two patients (8%) with recurrent disease required disease-modifying anti-rheumatic drugs (certolizumab, sulfasalazine) and spontaneous resolution without medical therapy have been reported (n=2).
Favourable outcomes of COVID-19 reactive arthritis were noted in all patients. The median follow-up period ranged from 1 week to 3 months. A follow-up with the rheumatology team is helpful in such patients to indicate laboratory, radiological, and clinical improvement. In general, reactive arthritis is a self-limiting disease and patients who are positive for HLA-B27 have a high-risk of chronic recurrent disease and sacroiliac joint involvement.
Viral arthritis is another entity reported to affect around 1% of patients with acute inflammatory arthritis.32 Fever, arthralgia, arthritis (monoarticular or polyarticular) and rash can develop during an acute viral infection. Various viral pathogens reported with different pattern of joints involvement such as parvovirus, Epstein–Barr virus, HIV, Chikungunya virus, Zika virus, and hepatitis B and C viruses.17 Acute arthritis diagnosed in patients with COVID-19 is uncommon. López-González et al.,33 reported 81 (26.4%) out of 306 patients with COVID-19 complained of arthralgia and myalgia at presentation. Four adult males (1.3%) developed acute arthritis (mean age: 60.25 years) and onset of arthritis ranged from 3–21 days. All the patients had prior history of recurrent crystals induced arthritis and they had a flare up of arthritis gout (n=3) and calcium pyrophosphate disease (n=1) during acute COVID-19 infection. The results of reverse transcriptase-PCR for SARS-CoV-2 RNA in synovial fluid were negative.33
AVN of bilateral femoral and multi-focal medullary bone infarctions involving right femur and both proximal tibias were noted in the authors’ patient. Such distinctive abnormalities were not reported in association with the COVID-19 infection prior to the authors’ case. There is compromised subchondral microcirculation leading to infarction of the bone due to various aetiologies. Systemic lupus erythematosus, alcohol, and sickle cell anaemia are among the differential diagnosis of AVN that were ruled out in the authors’ patient.
Corticosteroid use has been associated with 10–30% of AVN cases in retrospective studies.34 The use of corticosteroids in severe COVID-19 infection is supported by clinical trials.35 A prolonged use of corticosteroids in COVID-19 infection might predispose to AVN. Furthermore, the cumulative dose of 2,000 mg of prednisolone or its equivalent has been associated with AVN development.36 However, the authors’ patient received only a 5-day course of IV corticosteroids (cumulative dose: 200 mg) and had no other risk factors for AVN. Larger follow-up studies need to be conducted to understand the risks of AVN following corticosteroid use in patients with COVID-19 patients. In addition, a severe COVID-19 infection might increase the risk for AVN due to hypercoagulation caused by cytokine activation syndrome.37
It is possible that AVN and reactive arthritis in the authors’ patient were directly linked to the COVID-19 infection and exacerbated by an unidentified superimposed infection. The authors could not identify the organism causing pneumonia in the second hospital admission on sputum, blood, and respiratory viral panel PCR. The HLA-B27 positivity may been genetically predisposed the authors’ patient to develop reactive arthritis post-COVID-19 infection.
CONCLUSION
Reactive hip arthritis and AVN are rare MSK manifestations after a severe COVID-19 infection. HLA-B27 positive testing might indicate a severe and delayed form of arthritis with risk of recurrence. NSAIDs, either alone or combination with steroids, had favourable outcomes.
Larger studies are required to delineate the potential risk factors, clinical involvement, and long-term management outcomes for reactive arthritis and AVN associated with the COVID-19 infection.







