Abstract
The advances in treatments, including disease-modifying anti-rheumatic drugs and biologic agents, have significantly improved the management of inflammatory rheumatic diseases, allowing females with severe disease to become pregnant and lactate, previously considered as prohibited. Maintaining low disease activity with medications known to be safe from pre-conception to post-partum is a key point in reducing adverse pregnancy outcomes. Numerous observational and case studies have provided a growing amount of evidence on the use of safe anti-rheumatic medications in patients during pregnancy and lactation. Based on this information, this review discusses the safety of medications for patients with inflammatory rheumatic diseases during pregnancy and lactation. Among these, hydroxychloroquine, sulfasalazine, azathioprine, low-dose glucocorticoids, and low-dose aspirin are considered compatible with pregnancy, while methotrexate, cyclophosphamide, mycophenolate mofetil, and leflunomide are contraindicated. Non-steroidal anti-inflammatory drugs are only recommended for use early in pregnancy, as they are reported to cause rare but serious kidney problems in the fetus after 20 weeks or later. Cyclosporin, tacrolimus, and anti-TNF agents can be continued throughout pregnancy if the benefit is greater than the potential risk for the individual patient. Physicians should carefully weigh the risks and benefits of medications in patients with inflammatory rheumatic diseases considering pregnancy.
INTRODUCTION
The majority of inflammatory rheumatic diseases (IRDs) affect females more frequently and many have peaks in their childbearing age.1 Pregnancy can influence underlying disease activity by inducing a variety of changes in the hormone levels, type of immune responses, inflammatory cytokine signals, and interactions of molecular pathways.2 These changes may lead to an increased risk of disease flare during pregnancy and post-partum periods in IRDs.3 Females with IRDs hoping to conceive may have to consider the combination of worsening pregnancy by disease, disease flare by pregnancy, and the safety of medications during pregnancy and lactation.3 Therefore, pregnancy and lactation in fertile females with IRDs are always challenging.
Notably, over the past decades, significant improvements in the management of pregnancy have made it possible to maintain pregnant females with quiescent disease through safe medications. However, the actual adherence is known to be low, as previous studies in pregnant females with chronic diseases reported that approximately 40% of females do not adhere to their medications because of negative beliefs.4 In this regard, educational intervention by physicians is needed to reinforce the positive beliefs that appropriate medications can reduce adverse maternal and fetal outcomes. Physicians should be aware of professional and accurate information to determine the optimal timing of pregnancy, considering the potential benefits and risks of medications. In this article, the authors discuss the effects of commonly used anti-rheumatic medications on pregnancy and lactation and provide guidelines for the safe use of these medications (Table 1).
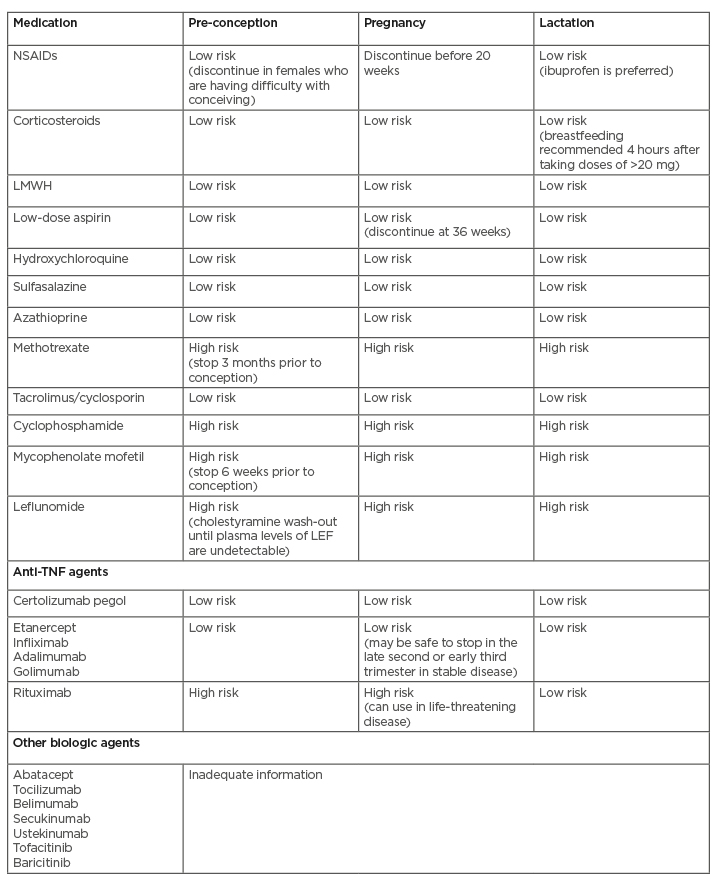
Table 1: Recommendations of anti-rheumatic medications in females during pregnancy and lactation.
LEF: Leflunomide; LMWH: low-molecular-weight heparin; NSAIDs: non-steroidal anti-inflammatory drugs.
ANTI-RHEUMATIC MEDICATIONS IN PREGNANCY AND LACTATION
Non-steroidal Anti-inflammatory Drugs
Non-steroidal anti-inflammatory drugs (NSAIDs) are the most commonly prescribed medication and are often used to treat fever, pain, and inflammation and can be easily available over the counter. Most experts agree that the traditional NSAIDs are probably safe to use in small to medium doses during the first trimester.5 While the majority believed that safety would be maintained throughout the second trimester, a warning recently issued by the U.S. Food and Drug Administration (FDA) added to the risk of NSAIDs in the second trimester. In October 2020, the FDA announced that the use of NSAIDs after 20 weeks of pregnancy increases the potential risk of fetal renal dysfunction, oligohydramnios, and neonatal renal impairment.6 In the third trimester, all NSAIDs are contraindicated as they have been linked with an increased risk of a premature closure of the ductus arteriosus and inhibition of labour.7,8 Moreover, the patients planning for pregnancy also need to avoid or reduce the use of NSAIDs, as they have been shown to significantly reduce fertility rates by inhibiting ovulation and reducing progesterone levels.9 While cyclogenase-2 (COX-2) inhibitors may potentially have the same side effects as other NSAIDs, they can be considered higher risk for pregnant females because one study showed that COX-2 inhibitor exposure increases musculoskeletal malformations.10
The majority of the NSAIDs are proven safe during lactation because they are poorly transferred to milk, and safety in children has been well studied; however, there are some reports of increased risk of jaundice and kernicterus.11,12 It is recommended to use relatively proven safe medications (i.e., ibuprofen) with extremely low levels in breastmilk and a short half-life.13 The data on the lactation of COX-2 inhibitors are limited.
Corticosteroids
Corticosteroids are used to treat a wide range of IRDs, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), inflammatory myopathy, and other connective tissue diseases, and are often necessary to control the activity of the disease during pregnancy. They are considered the first-line therapy for acute flares throughout pregnancy because of a rapid onset of action. Important to recognise is that the types of corticosteroids vary depending on whether the treatment target is the mother or the fetus. Corticosteroid treatment for mothers requires short-acting agents (prednisone, prednisolone, and methylprednisolone), and 90% is metabolised by the placental enzyme 11β-dehydrogenase.14 If corticosteroid treatment is needed for the fetus, dexamethasone and betamethasone, which have the ability to cross the placenta from the mother to fetus, should be chosen. Corticosteroids need to be delivered to the fetus to prevent complete congenital heart block in neonatal lupus syndrome and induce lung maturity in preterm labour.15,16
Although corticosteroids are generally considered as safe medications during pregnancy, side effects can occur in both the mother and fetus. In terms of maternal complications of corticosteroids, pregnancy-specific complications such as pre-eclampsia, gestational diabetes, and premature rupture of membranes may occur.17
Several studies have shown an increased incidence of cleft palate and intrauterine growth retardation following corticosteroid therapy.18 Given the potential risk of complications, it is preferable to keep the corticosteroid dose below 5–10 mg/day.19
Prednisone and prednisolone pass into breastmilk in small quantities and are safe medications during lactation. Up to 20 mg of maternal prednisolone is not expected to cause any adverse effects on breastfed infants. For mothers taking high-dose corticosteroids, it is advised to breastfeed 4 hours after taking them to minimise drug exposure.20 There are no data available on the use of dexamethasone or betamethasone during lactation.
Antiplatelet and Anticoagulant Agents
Antiphospholipid syndrome (APS) is a systemic autoimmune disorder characterised by recurrent vascular thrombosis or fetal loss in the presence of antiphospholipid antibodies (aPL). Without treatment, pregnancy complications increase to 90%; however, it is widely accepted that the miscarriage rate can be greatly improved through anticoagulation treatment.21 The standard anticoagulation treatment for APS is lifelong anticoagulation with warfarin or an alternative vitamin K antagonist. In particular, since pregnancy is related to a hypercoagulable state, females with APS need careful attention throughout the pregnancy and post-partum period. Notably, warfarin is a teratogen that can cross the placenta and should be avoided between 6 and 12 weeks of gestation.22 Patients with APS undergoing warfarin treatment are recommended to confirm pregnancy 6 weeks before the conversion of anticoagulation from warfarin to a combination of low-molecular-weight heparin (LMWH) and low-dose aspirin (LDA). LMWH and LDA have not been detected to exert any specific adverse fetal side effects or teratogenicity.23 It is suggested that the same dose of LMWH should be continued for 6 weeks after delivery, as the risk of post-partum thrombosis may increase. In the case of asymptomatic aPL carriers (those with positive aPL but no vascular thrombosis or pregnancy complications), LDA helped protect pregnant females from thrombosis.24 Both LMWH and LDA are not well excreted into breastmilk; therefore, breastfeeding is safe in mothers who are treated with both drugs.23
Despite the widespread use of direct-acting oral anticoagulants for the prevention of secondary thrombosis in the general population, it is not recommended to be used in patients with definite APS.24 No clinical trials have been performed on direct-acting oral anticoagulants during pregnancy and lactation, and safety has not been demonstrated during this period.25
Conventional Disease-Modifying Anti-rheumatic Drugs
Hydroxychloroquine
Hydroxychloroquine (HCQ), an antimalarial drug, has been widely used, either alone or in combination with other agents, in the treatment of SLE, RA, and other IRDs. Above all, in patients with SLE, HCQ should be continued during pregnancy in order to prevent a disease flare and reduce adverse pregnancy outcomes. Despite the theoretical concerns of retinal toxicity and ototoxicity, generally known as HCQ toxicity from long-term use, no visual, auditory, or congenital abnormalities have been reported in children in previous studies.26 One study suggested that the continuation of HCQ during pregnancy has a possible protective effect against the occurrence and recurrence of neonatal lupus and congenital heart block.27
HCQ also appeared to be compatible with breastfeeding. Low concentrations of HCQ can be found in breastmilk and exposed to infants; however, there are no data on the adverse effects of breastfeeding from mothers taking HCQ till date.28
Sulfasalazine
Sulfasalazine (SSZ) is a compound of 5-aminosalicylic acid and sulphapyridine developed for the treatment of RA more than 60 years ago. Aside from its use as a treatment for RA, ankylosing spondylitis, psoriatic arthritis, juvenile arthritis, and ulcerative colitis are also indications for treatment. Significant information about the risk of SSZ during pregnancy was obtained from patients with inflammatory bowel disease, and most studies have demonstrated safety during pregnancy.29 Although SSZ and its metabolite sulphapyridine pass through the placenta, current studies have shown that these concentrations do not cause significant displacement of bilirubin from albumin.30 The risk of kernicterus does not appear to increase in infants exposed to SSZ. It should be noted that folate supplementation is necessary during pre-conception and pregnancy, as SSZ is a potential inhibitor of folate carrier.31
There is a general consensus that SSZ is safe during lactation. However, there has been one reported case of bloody stool and diarrhoea in an infant receiving breastmilk from mothers taking SSZ; thus, it should be explained to patients to observe the symptoms and signs of infants treated with SSZ throughout this period.32
Azathioprine
Azathioprine (AZA) is a purine analogue, an immunosuppressive agent that prevents T- and B-cell proliferation by inhibiting nucleic acid synthesis.33 Teratogenicity has been found in animals due to DNA damage caused by the active metabolites of AZA, but it has not been identified as a human teratogen because there is no enzyme that converts into active metabolites in the fetal liver.34 In the past, numerous case series have been reported for adverse outcomes of pregnancies, including intrauterine growth retardation, chromosomal anomalies, and immunosuppression; however, there are limitations in the analysis of results, given that a small number of patients were included and that the investigators did not consider the severity of the disease.35 Based on the safety data during pregnancy, proven primarily from observational studies of inflammatory bowel disease and organ transplantation patients, AZA has recently been recognised as a safe immunosuppressant and steroid-sparing agent in pregnant females with various IRDs.36 Breastfeeding is compatible with AZA as the drug transfer into maternal milk is minimal.37
Methotrexate
Methotrexate (MTX) is a folate antagonist that inhibits dihydrofolate reductase and causes the termination of cell growth and division. Although it is commonly used for autoimmune diseases such as RA and psoriasis because of its anti-inflammatory effects, MTX is a teratogen and abortifacient by inhibiting folic acid, which is essential for the development of fetal neural tubes.38 The major congenital malformations induced by MTX include microcephaly, hydrocephalus, cleft palate, congenital cardiopathy, and delayed ossification.39 The critical period of malformation production by MTX is regarded as 6–8 weeks after conception.40 Since MTX can persist in the maternal liver up to 4 months after exposure, females who wish to conceive should stop taking the medication for at least 3 months before attempting pregnancy.17 Considering the widespread use of MTX in rheumatic diseases, physicians should inform females of childbearing age regarding the risks of taking this medication and continue to recommend folic acid supplementation. Most expert guidelines suggest that breastfeeding is contraindicated during maternal MTX treatment.41 Although the levels found in breast milk are very low, they can accumulate in neonatal tissues.42
Tacrolimus and cyclosporine
Tacrolimus (TAC) and cyclosporine (CSA) are calcineurin inhibitors that interfere with the transcription of IL-2 and several other cytokines in T lymphocytes.43 Both medications belong to a family of immunosuppressive agents and are widely used in the prevention of transplant rejection and in the treatment of autoimmune disorders. The current available data indicate favourable pregnancy outcomes without any evidence of increased risk of congenital anomalies following intrauterine exposure to TAC.44 CSA is also classified as a relatively safe medication during pregnancy, although the incidence of maternal diseases such as pre-eclampsia, gestational diabetes, and maternal hypertension may increase.45 The medical literature concerning the effects of TAC and CSA on lactation is not known precisely as it mainly includes case reports and registry data. TAC and CSA are considered safe as the detectable concentration in breast milk is extremely low and there are no cases of increased malformations in pregnant females exposed to these medications.46
Cyclophosphamide
Cyclophosphamide (CYC) is an alkylating agent that is toxic to cancer cells and proliferating lymphocytes and is known as a treatment for various rheumatic diseases such as SLE and vasculitis as well as malignancies.47 This medication is generally avoided in females of reproductive age because of its impact on embryofetal toxicity and fertility. CYC administration to pregnant females not only produced deformities of the skeleton, limbs, eyes, and palate, but has been observed to result in severe bone marrow hypoplasia, gastroenteritis, and fetal resorption. The risk seems to increase among the fetuses exposed during the first trimester.48 For this reason, during pregnancy planning, CYC should be discontinued before conception and switched to pregnancy-compatible medications to avoid fetal exposure to medication. Considering the risk of ovarian failure, other immunosuppressive agents should be preferred as first-line therapy for young females instead of CYC. Nevertheless, if the benefits of CYC outweigh the clinical risks, the use of gonadotropin-releasing hormone agonists during CYC therapy will help preserve ovarian function.49
Patients taking CYC should avoid breastfeeding, as it appears in maternal milk in potentially toxic amounts. There are reports that breastfeeding by females exposed to CYC caused neutropenia and bone marrow suppression in the infant.24
Mycophenolate mofetil
Mycophenolate mofetil (MMF) is an immunosuppressive agent that selectively inhibits the proliferation of lymphocytes by blocking purine synthesis in B and T lymphocytes.50 It has become a major treatment for lupus nephritis and other IRDs, with fewer side effects on fertility, bladder toxicity, cancer, and infection compared to CYC. Apart from the evidence that MMF is not related to the risk of infertility, this medication is widely known to increase the risk of congenital malformations and miscarriage during pregnancy if the fetus is exposed in utero.51 Toxic effects include facial cleft; anomalies of the external ear, vertebra, rib, eye, and intestine; congenital heart disease; and 2–3 times higher rates of miscarriage than non-exposed groups.51 Since there have been many cases of malformation occurring, especially when exposed to MMF during the critical organogenesis period, the manufacturer recommends effective contraception for at least 6 weeks after last treatment.52 There is a paucity of information regarding the transmission of MMF into breast milk; therefore, breastfeeding should be discouraged during treatment with MMF.
Leflunomide
Leflunomide (LEF) is a potent inhibitor of pyrimidine nucleotide synthesis and protein tyrosine kinase, which is mainly used as a treatment for RA. Embryonic cells require a large amount of nucleotides for DNA and RNA synthesis to proliferate and, in this respect, LEF may be embryotoxic.53 In animal studies, LEF reduced the fetal viability and increased the incidence of exencephaly, cleft palate, and deformities of the skeleton, heart, and vessels at therapeutic doses, similar to those used in humans.54 Hence, LEF is contraindicated during pregnancy and lactation. LEF has a long half-life and enterohepatic circulation that can be sequestered in the bile circulation for up to 2 years after drug cessation. In the event of unintended pregnancy, the drug should be eliminated for 11 days with 8 g cholestyramine three times daily; afterward, plasma levels <0.02 mg/mL should be verified twice at least 2 weeks apart.53
Biologic Disease-Modifying Anti-rheumatic Drugs
Anti-TNF therapy
Anti-TNF agents have been developed as treatments for autoimmune diseases, as TNF is deemed a master pro-inflammatory cytokine that is a major cause of autoimmune inflammation.55 To date, five agents have been approved for IRDs, including etanercept, infliximab, adalimumab, golimumab, and certolizumab pegol. Most studies generally suggest that anti-TNF therapy has a lower risk in pregnancy, especially in the first two trimesters.56 It is necessary to weigh the benefits and potential risks of maintaining anti-TNF agents in consideration of disease activity. Although slightly different depending on the drug, it may be safe to stop treatment in the late second or early third trimester in stable disease, as anti-TNF agents can pass through the placenta after 20 weeks. On the other hand, treatment with an anti-TNF agent is an appropriate option for females with severe active diseases to have a successful pregnancy. Breastfeeding is also compatible with anti-TNF therapy. Among them, certolizumab pegol is known to be the safest during pregnancy and lactation. It is notable that neonates exposed to anti-TNF agents should avoid live vaccinations during the first 6 months of life.57
Rituximab
Rituximab (RTX) is a chimeric monoclonal B-cell depleting anti-CD20 antibody indicated for RA, SLE, systemic sclerosis, anti-neutrophil cytoplasmic antibody-associated vasculitis, and inflammatory myopathy.58 According to the analysis of neonates who were exposed to RTX, few congenital malformations or neonatal infections were observed.59 Nevertheless, the routine use of RTX in females who plan to conceive or become pregnant is discouraged. Although the offspring of pregnant animals exposed to RTX had no teratogenicity, lymphoid B cells were depleted in the newborn, and in humans RTX was detected in the serum of infants after intrauterine exposure.60 Except for potentially life-threatening diseases occurring during pregnancy, all females of childbearing age should continue to be counselled to avoid pregnancy for ≥12 months after RTX exposure.
There are no data on whether RTX is transmitted to maternal milk and its effect on breastfed children. The European League Against Rheumatism (EULAR) and American College of Rheumatology (ACR) argue that this medication is considered safe because of its large molecular weight and poor absorption into breast milk.41,56
Other biologic agents
Scant data are available regarding the compatibility of other biologics with pregnancy and lactation. CTLA-4 inhibitors (i.e., abatacept), IL-6 inhibitors (i.e., tocilizumab), B-cell activating factor inhibitors (i.e., belimumab), IL-17 inhibitors (i.e., secukinumab), and IL-12/23 inhibitors (i.e., ustekinumab) are included in this group. They are conditionally recommended before conception, in that these agents do not cross the placenta until the second trimester, but should be stopped during pregnancy. Further investigation is expected, given that there are little data on the effects of these biologic agents during pregnancy and lactation.
Targeted Synthetic Disease-Modifying Anti-rheumatic Drugs
JAK inhibitors, a ‘target’ therapy that acts on the immune response like other biologic agents, are the latest approved medication for use as a treatment for RA.61 Currently, these agents include two JAK inhibitors, tofacitinib and baricitinib, which block one or more JAK enzymes and prevent the signalling of pro-inflammatory cytokines.
As they are a small molecule that can cross the placenta and are known to decrease the fertility and increase embryo lethality in animal models, the randomised controlled trial protocols excluded pregnant females.62 Case reports with no adverse outcomes of pregnancy in females exposed to JAK inhibitors are extremely rare; however, there is little information on the safety of these medications during pregnancy so far.63 Therefore, the present recommendation is that the use of JAK inhibitors should be discontinued during pregnancy and lactation.
MALE FERTILITY AND RISK OF CONCEPTION WITH ANTI-RHEUMATIC THERAPY
Assessment of the safety of paternal exposure to medications during pregnancy planning considers whether the medication causes infertility or can lead to adverse pregnancy outcomes. Except for CYC and SSZ, most medications are not teratogenic and do not affect fertility.64 CYC is reported to lead to irreversible azoospermia and potential teratogenicity and should be discontinued for 3 months prior to conception. SSZ causes reversible azoospermia; thus, it is recommended that males who take this medication and have difficulty conceiving are advised to stop this medication for 3 months. Table 2 summarises the safety of paternal exposure to anti-rheumatic medications.
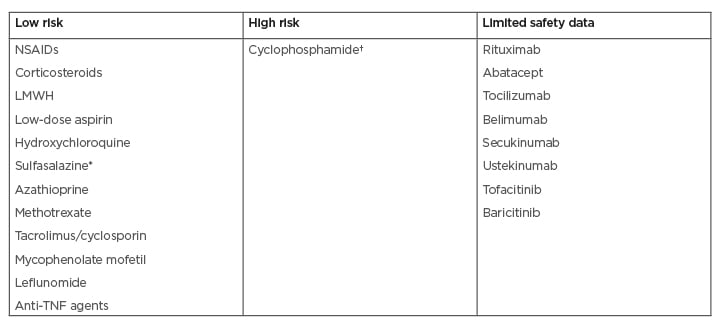
Table 2: Safety of anti-rheumatic medications in males during pregnancy planning.
*Sulfasalazine can reduce the counts and motility of sperm, causing reversible azoospermia at doses >2 g/day.
†Cyclophosphamide has a high risk of irreversible infertility at doses >7.5 g.
LMWH: low-molecular-weight heparin; NSAIDs: non-steroidal anti-inflammatory drugs.
CONCLUSION
The authors have discussed the safety of medications during pregnancy and lactation in patients with IRDs. Prior to the initiation of treatment for females with rheumatic diseases of childbearing age, extensive counselling should be provided on the possible toxicity of exposure to medications and the potential risks should be emphasised in the case of an unplanned pregnancy. Physicians should carefully weigh the risks and benefits of adverse outcomes during pregnancy from exposure to the medication against disease flare when withholding maternal treatment.







