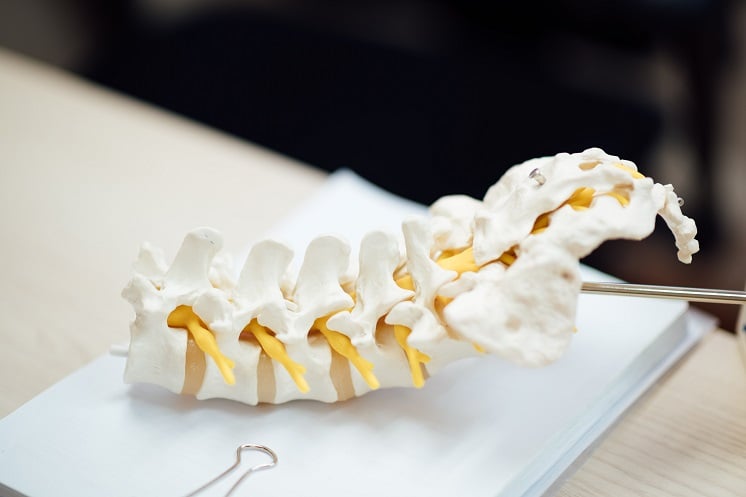
ANKYLOSING spondylitis (AS) is an early on-set inflammatory condition that affects the spine and other parts of the body. Sections of the spine can begin to fuse reducing mobility and causing back pain. Currently, there is no cure for AS and treatment involves physiotherapy, exercise, and pain medication.
The researchers explored the efficacy of upadacitinib in treating AS. Upadacitinib targets the inflammatory pathway and works by inhibiting JAK, reducing the ability of JAK to cause inflammation in the joints.
In a recent placebo-controlled clinical trial, named the SELECT-AXIS 1 study, researchers aimed to test the efficacy and safety of upadacitinib in patients with AS. In this study, N=187 patients with AS who had an inadequate response with non-steroidal anti-inflammatory drugs were randomised to be given either 15 mg of upadacitinib once a day or a placebo for 14 weeks. Once the patients had completed this round, they were offered to apply for an extension, and they received open-label upadacitinib for up to 104 weeks. In total, 178 patients had completed the first part of the trial, 89 continued to receive upadacitinib, and another 89 patients began to take upadacitinib.
The results showed that there was a significant difference between patients receiving upadacitinib and patients given a placebo. A significantly greater number of patients receiving upadacitinib achieved Assessment of SpondyloArthritis International Society 40 (ASAS40), where there is at least a 40% improvement, compared to placebo. Further to this, out of the 618 adverse events reported there were no serious infections or cardiovascular events.
Scientists were pleased with the promising results and believe upadacitinib might provide an effective alternative for patients who cannot take NSAIDs. Further research of the long-term use of this drug is needed and the scientists will move on to assess upadacitinib over 2 years.