Meeting Summary
With an increasing number of biosimilars receiving regulatory approval, the treatment landscape for rheumatic diseases is evolving. Healthcare professionals (HCP) are being presented with an expanding armamentarium of treatment options that can increase patient access to effective biologic therapies and offer an opportunity for healthcare systems to benefit from significant cost savings. In recent years, accumulation of clinical and real-world data with biosimilars has helped physicians gain confidence in the use of biosimilars in daily clinical practice. However, further information regarding best practices of how to effectively introduce biosimilar therapies into a busy clinic is still required. This symposium aimed to uncover various aspects of preparing for a switch, providing suggestions for clinical parameters and imaging tools to aid identification of patients who will respond optimally to biologic treatment. Additionally, HCP–patient communication was analysed from a psychosocial perspective, covering shared decision-making and how to appropriately address common concerns raised by patients. Finally, during this interactive session, country-specific perspectives on best practices for successful switching and the use of remote monitoring tools for patient follow-up were also discussed.
Introduction: The Treatment Landscape of Rheumatic Diseases
Anti-TNF therapies have revolutionised the treatment of rheumatoid arthritis (RA) and a number of other inflammatory diseases, including psoriatic arthritis, juvenile arthritis, Crohn’s colitis, axial spondyloarthritis (axSpA) including ankylosing spondylitis, and psoriasis. However, the high cost of these agents has placed a significant burden on healthcare systems around the world. Between the first anti-TNF biosimilar receiving approval in 2013 and the anticipated arrival of adalimumab biosimilars from late 2018, the numerous biosimilars currently licensed in Europe have presented HCP with an opportunity to increase patient access to effective biologic treatments in a cost-effective manner. “The emergence of a large number of biosimilars in the past few years, namely for rituximab, infliximab, and etanercept reference products, has led to significant health-economic benefits, with savings estimated to be between €12 billion and €33 billion globally between 2007 and 2020.1 Switching patients to biosimilars creates opportunities for very significant savings in the clinic, which can then be reinvested to benefit patients further,” highlighted Prof Taylor.
The increasing level of clinical, registry, and real-world evidence has reinforced confidence in the effectiveness and safety of biosimilars, leading European regulatory authorities and drug associations to endorse the interchangeability between biosimilars and reference biologics. However, despite their confidence in initiating biologic-naïve patients, HCP remain unsure about the practical aspects of switching their patients from a reference biologic to a biosimilar: which patient should be switched, when, and how? Are there ways to select patients that will benefit from biosimilar treatment? How should a switch be communicated to a patient? Following a successful switch, is there a way to monitor the patient without burdening the practice or the patient? To help address these questions, the expert faculty and the audience discussed key considerations when switching patients to a biosimilar treatment, as well as follow-up methods currently available to help optimise patient management and ensure maximum treatment outcomes.
Identifying Patients Who Will Benefit Most from Biologic Treatment
Switching should not be perceived as a burden when considering a patient for biologic therapy; instead, this is a prime occasion to reassess the clinical and psychosocial status of a patient. Together, these assessments can help identify patients who will benefit the most from biologic therapy and help HCP prepare patients for a change in therapy. Similarly, these evaluations can be applied to support patients switching from a reference biologic to a biosimilar to ensure that they experience an optimal response to the biosimilar. Here, Dr Sengupta and Prof Braun provided insights into which clinical and imaging criteria they consider when assessing patients with RA or axSpA for a switch to a biosimilar. Dr Sengupta highlighted: “Picking the right patient is possible and can bear real fruit, as most patients continue on the biosimilar when they are responding clinically.”
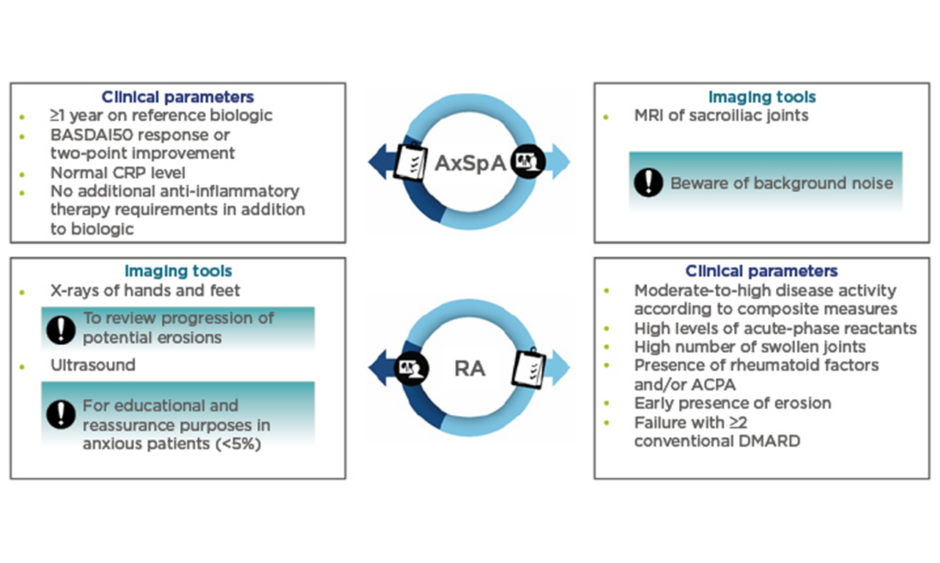
When considering patients with axSpA, Dr Sengupta believes that it is important to select patients who are doing well on a reference biologic. The key clinical parameters used by Dr Sengupta can be found in Figure 1. In addition, he advised prudence when considering a switch for patients who are planning pregnancy or surgery, or for those who present with concurrent fibromyalgia.
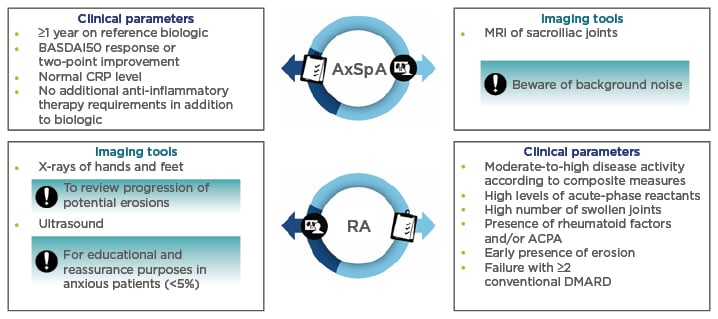
Figure 1: Clinical parameters and imaging tools to guide selection of patients with rheumatoid arthritis and axial spondyloarthritis for a switch to a biosimilar.
ACPA: anticitrullinated protein antibody; AxSpA: axial spondyloarthritis; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; CRP: C-reactive protein; DMARD: disease-modifying antirheumatic drug; RA: rheumatoid arthritis.
Concerning imaging techniques, Prof Braun cautioned that in all patients with chronic inflammatory rheumatic diseases, such as axSpA, it is important to use imaging techniques (such as MRI) correctly to accurately define disease activity and help identify patients who are responding well to the reference biologic. With the occurrence of severe sacroiliitis, presenting with a large bone marrow oedema on MRI, and human leukocyte antigen-B27 being predictive of the development of ankylosing spondylitis, Prof Braun advised that there is value in obtaining an MRI of the sacroiliac joints. This can be used not only for diagnosis or classification but also for the assessment of disease activity at baseline and follow-up, as well as for prognostic purposes. However, it is important to correctly interpret the MRI changes because it is known that at least some of the changes may also occur in healthy individuals.2-4 “Reference biologics, such as anti-TNF therapy, have been shown to be effective in targeting inflammation, whether in the sacroiliac joints, the spine, or the peripheral joints such as the knee. Within 6–12 months, the inflammation is usually cleared,5 but in up to 20% of cases, a follow-up MRI may reveal some residual inflammation. The significance of this finding is, however, unclear, especially as the correlation to the clinical finding is reportedly marginal.5 Since no studies have been performed in this area, it is impossible to make a recommendation; however, it appears that these patients could respond less well if switched to a biosimilar. In any case, it is the clinical response that finally counts,” commented Prof Braun.
Several studies2-4 have recently suggested that a positive MRI suggestive of axSpA according to the Ankylosing Spondylitis Disease Activity Score (ASDAS) definition6 is frequently found in individuals who do not have axSpA. Thus, there is considerable background noise that is not indicative of axSpA, with MRI scans appearing positive in 6.4% of patients with chronic back pain, 57.1% of post-partum females, and 12.5% of runners, as well as 23.4% of healthy volunteers.4 Prof Braun further explained that “although lesions are most frequently indicative of axSpA, one should look at the complete clinical picture and not make a diagnosis based on classification criteria alone. What may help to distinguish axSpA from non-axSpA patients are the deep lesions characterised by a large bone marrow oedema present in several slices.” In addition, Prof Braun encouraged the use of the ASDAS:6 “It provides very clear cut-off points that can really help you differentiate between inactive, moderate, high, and very high disease activity, as well as treat-to-target recommendations to help you combat inflammation and prevent structural changes.”
Similarly to patients with axSpA, patients with RA who are responding well to the reference biologic treatment may benefit from a switch to a biosimilar. According to Prof Braun, clinical parameters used to assess disease activity should include prognostic factors that are predictors of poor outcomes (Figure 1).7 On that basis, therapy should be started as early as necessary to induce remission. In terms of imaging techniques, Dr Sengupta primarily discussed the use of X-ray and ultrasound (Figure 1): “If X-rays of hands and feet show clear progression of erosions, [the HCP] should investigate if the patient is taking the medication correctly or where the issue lies before considering switching the patient.” Similarly, ultrasound has been shown not to have an additional diagnostic value in routine practice8 but, according to Dr Sengupta, may have an educational role in symptomatic patients without clinical signs of active disease by reassuring them that their disease is controlled.
Re-evaluating Healthcare Provider–Patient Communication
Evaluating clinical and imaging parameters can help assess disease state prior to considering a switch. Patients expected to maintain a good treatment response when switched to a biosimilar should then be introduced to the switch through shared, informed decision-making. For this, HCP should tailor their patient communication to meet individual patient needs and build their confidence in the treatment. This will not only affect the patient’s perception of the biosimilar but will provide the patient with the tools to maximise treatment outcomes. As Dr Sengupta highlighted: “It is crucial that the patients accept what is about to happen, as this increases the possibility of a successful switch. We might debate the pathway and how we get the patient’s acceptance, either via a letter and/or face-to-face discussion, but the principle is that the patient has to understand that the biosimilar will be as effective as the reference biologic.” Prof Enck therefore encouraged HCP to consider the importance of the patient relationship: “The prescribing physician can have a significant impact on the perceived pharmacological effect of a drug. Communication is much more important than anything you will prescribe to the patient, as it is your communication that drives what the patient will report, regardless of the drug.”
Prof Taylor noted that “each physician works in a very different context in which shared, informed decision-making, although ideal, may or may not be an option. However, in each situation there may be patient anxieties that we as physicians have a responsibility to address in a positive, constructive, and compassionate manner.” So, how should a HCP tackle patient communication when switching? According to Prof Enck, “no size fits all but there are certain dos and don’ts that should be considered (Figure 2); we should aim to use these appropriately in different settings to ensure that the correct message is conveyed to the patient in the right way, taking an average of 5–10 minutes, and therefore adhering to the time constraints of a busy clinic. In essence, we are talking about shared decision-making. If you [the HCP] make the decision to switch a patient but are not convinced yourself, then you cannot convince the patient that biosimilars are the best treatment choice. Only if you discuss the options with the patient and tell them what is best for whom, for society, for you as a HCP, and for the patient, then it becomes shared decision-making. Biosimilars are one of the options patients should take; they are the medicine of the future.” Accordingly, Prof Braun highlighted that “if the patients had to pay themselves, then the discussion would be very short. I always make three points: the biosimilar is a good drug, it has been tested heavily, it is almost the same, it does not have any clinically meaningful differences compared to the reference biologic; the biosimilar is a very effective drug; and it is good for society as it allows you [the HCP] to treat as many patients as possible; you [the patient] have an opportunity to contribute to that.”
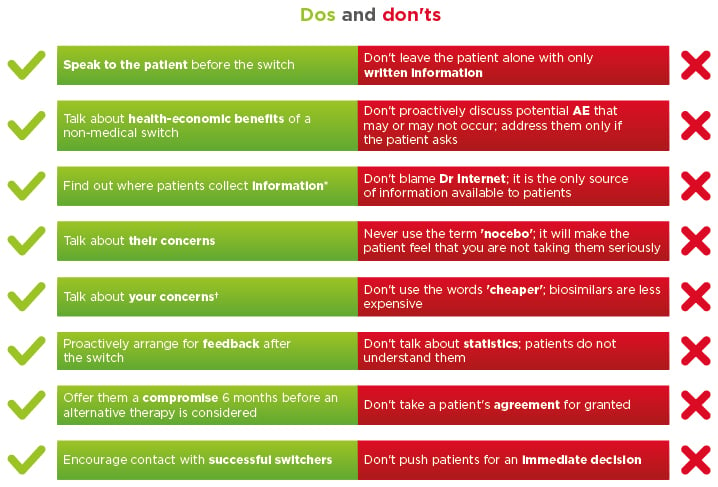
Figure 2: Dos and don’ts of healthcare professional–patient communication.
*More side effects are reported by patients who use the internet. †Costs or pressure from health insurance or representatives.
AE: adverse effects.
Furthermore, the nocebo effect is a topic of interest when discussing best practices; the nocebo effect is the negative equivalent of the placebo effect and has been shown to negatively influence treatment adherence and outcomes in multiple pathologies.9 Prof Enck highlighted that “the nocebo effect is not unique to reference biologic-to-biosimilar switch but occurs in all medical specialities, whether switching from a branded to a generic drug or from one drug to the next. Patients are always reluctant and sceptical about change.” HCP should therefore not automatically dismiss patient-reported adverse events (AE) as being a nocebo effect; it is important to acknowledge the symptoms appropriately.
The use of an information leaflet or letter to replace or support the prescribing physician’s face-to-face consultation when switching a patient was comprehensively debated among the faculty. Patients may not retain the information provided during the consultation and instead seek information independently. Providing an information leaflet or letter has successfully supported switches in Prof Taylor’s and Dr Sengupta’s practices in the UK: “Written information should be simple, short, and clear, giving the patient a rationale for the switch, both from the clinical and the health-economic perspective, as well as provide contact details for the patients to use in case of queries or concerns,” clarified Dr Sengupta. In comparison, Prof Braun felt that the use of a letter in his practice in Germany would be insufficient from a legal perspective and should be supplemented with verbal communication in the practice in order to be effective and safe. To address this need, the German Ministry of Health is investing in the education of nurses specialising in rheumatology and in structured patient education programmes. According to Dr Sengupta, communication via social media channels could also be an additional resource: “The younger patients definitely use social media, helplines, and fora. Collaborating with national charities can ensure that correct information is available online, helping patients understand the concept of biosimilars.”
Patients may react differently to the idea of switching. However, according to Dr Sengupta, the most frequent patient query is why they should switch when they are doing well on the reference product. Both Dr Sengupta and Prof Braun suggested that an honest approach should be used: “Explain the available studies and show the patient that the biosimilar has been tested extensively and it is as clinically effective as their current treatment. Reassure them that you believe they will maintain a good response. Discuss the societal benefits of switching, how these drugs are less expensive and will allow more patients to be treated with effective therapies,” explained Dr Sengupta. In agreement, Prof Enck added: “When switching to a biosimilar, it is not necessary to start the discussion about AE unless the patient initiates the conversation; from what we know, the biosimilars are very similar to the reference product and singling them out by talking about AE will just increase the patient’s anxiety, causing the patient to expect the AE.” Similarly, Dr Sengupta suggested avoiding priming the patient by telling them that they will be given an opportunity to switch back to the reference product: “This is not something I would standardly bring up. If the patient asks, I do provide assurance that we can.”
Successful Switching
Successful switching practices will vary across different countries, healthcare systems, and individual practices. Both Dr Sengupta and Prof Braun agreed that following a simple set of guidelines (Figure 3) was effective in their clinical centres, resulting in 90% of their patients with RA and axSpA remaining on biosimilar treatment post-switch. Dr Sengupta also confirmed that disease activity scores remained comparable between pre and post-switch patients. Also, in Norway, where the tender system is in effect, Prof Haugeberg reported that only 10% of patients requested to switch back to the reference biologic, suggesting that the use of a non-medical switch, whereby HCP are encouraged to prescribe a biosimilar as a matter of course, has not significantly affected treatment adherence rates.10
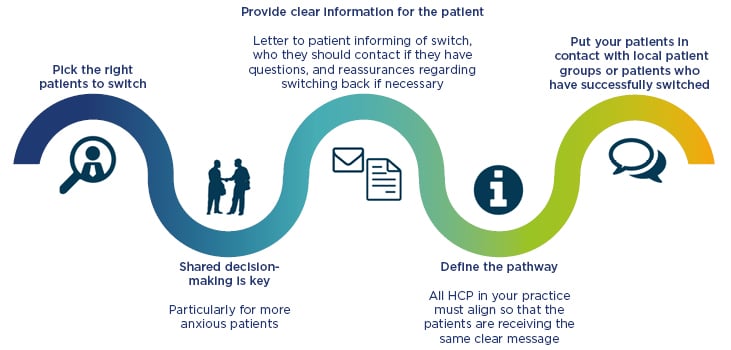
Figure 3: A pathway to successful switching.
HCP: healthcare professional.
Post-Switch Monitoring
Prescription of any biologic therapy requires careful follow-up to ensure that the treatment is safe and effective. Follow-up visits can enable patients to address any concerns or queries they may have. However, as Prof Haugeberg explained, as disease control improves, frequent monitoring of patients in-clinic may become redundant: “In Norway, between 2004 and 2013, the proportion of RA patients in remission increased from 20% to 55%. This caused us to rethink how we monitor patients: do they all need to take time out in their busy schedule to attend a clinic appointment, only to be told they are fine?”
This question motivated Prof Haugeberg to participate in the initiation of an app, a remote monitoring tool currently being tested in Norway. Prof Haugeberg explained: “Patients need a simple tool with brief questions that they can answer at their own leisure in a place of their choice, which they can use to report how they feel. This can be done through a smartphone app that prompts the patient to regularly give simple feedback of well-known patient reported outcome data to the physician or nurse. This system can also support patients in deciding when they should be requesting follow-up appointments or when the monitoring can continue online. This has the potential to free up a significant amount of time for the clinic and shortens the waiting lists for an appointment. It also allows us to monitor the patients remotely.” To be compliant with data privacy rules, a remote monitoring tool needs to be securely encrypted and anonymised appropriately. Patients need to retain control of their personal data and the ability to request its deletion. “In Norway, we have a whole separate department within the health authorities to ensure compliance with all data protection laws,” commented Prof Haugeberg.
Conclusion
Confidence in biosimilars has grown alongside the volume of robust clinical trials and real-world evidence that has become available. Combined with the potential for significant cost savings, biosimilars offer healthcare systems the opportunity for sustainable treatment of rheumatic conditions. Furthermore, the endorsement of reference biologic-to-biosimilar switching by regulatory bodies in Europe has shifted the discussion towards questions around best practices for the effective implementation of biosimilars in the clinic. Here, key experts discussed clinical, imaging, psychosocial, and monitoring practices that can be used to ensure an optimal treatment response when switching a patient to a biosimilar.
When switching is not compulsory, careful selection of stable patients suitable for switching, by accurately measuring prognostic factors using both clinical and imaging tools, was advised. HCP–patient communication was deemed vital to an effective switch, with the HCP’s confidence in the biosimilar and the use of shared decision-making considered essential aspects in the process. Finally, regarding evolving technologies, online platforms and apps have provided significant opportunities for remote monitoring, bringing a new element to clinical management and assessment of disease remission. Simple remote monitoring tools could free significant resources for the practice, while allowing the HCP to follow a patient without either being constrained by clinical appointments. Prof Taylor concluded: “The faculty has provided very thoughtful comments that need to be applied in the cultural context in which each of us works. It is not necessarily the case that one approach to communication will suit everybody, but it is the case that we have the potential for very significant health-economic savings in the environment that we live in.”