Meeting Summary
This symposium took place during the 2019 European League Against Rheumatism (EULAR) congress in Madrid, Spain, and focussed on the unique challenges facing women with axial spondyloarthritis (axSpA) and psoriatic arthritis (PsA), highlighting differences in diagnosis, disease course, and treatment response between men and women.
Compared to men, women have a longer delay to axSpA diagnosis, higher disease activity, lower quality of life, and experience more fatigue, peripheral involvement, and functional impairment, despite less radiological damage and a lower treatment response to biologicals. In addition, axSpA in general is associated with depression, anxiety, reduced work productivity, and an increased risk of adverse pregnancy outcomes.
Women with PsA typically present with a higher number of involved joints than men, poorer patient-reported outcomes, and a lower quality of life. They also report higher disability scores, more fatigue, a higher prevalence of depression, and often delay or abandon decisions to start a family or to breastfeed their infants. Although a treat-to-target approach is endorsed by both EULAR and Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) guidelines for the management of PsA, minimal disease activity (MDA) is less frequently achieved by women compared to men.
Biologic anti-TNF drugs are efficacious in both SpA and PsA. However, during pregnancy and breastfeeding, most anti-inflammatory biologics used for the management of PsA and SpA are not recommended because of the risk of drug transfer across the placenta to the fetus or via the breastmilk to the infant. Exceptions are the TNF inhibitors adalimumab and certolizumab pegol, a PEGylated Fab’ fragment of a humanised monoclonal antibody, for which use in pregnancy and breastfeeding has been documented by clinical and registry data.
In conclusion, efficacious treatment strategies do exist that allow women with axSpA or PsA to achieve satisfactory disease control, also during pregnancy and when breastfeeding.
Sex and Gender Differences in Axial Spondyloarthritis and Psoriatic Arthritis
Professor Irene van der Horst-Bruinsma
Although gender is defined by psychological and social differences between men and women, and sex is defined by biological parameters based on genetics, anatomy, and physiology,1 the term ‘gender’ will be used throughout this symposium report to represent both sex and gender differences. In humans, gender is genetically determined by the X and Y chromosomes, and although the male and female versions of the human genome differ in only a limited number of genes located on either, differences in gene expression between men and women are distributed across the entire genome and not only focussed exclusively on the X and Y chromosomes.2
Additionally, differences between men and women are often not considered in drug development, as drugs are predominantly tested in healthy male volunteers, with no correction of dosages for body weight and gender, or correction for gender in post marketing studies (Figure 1).3 Other differences between men and women that may impact the effectiveness and safety of drugs in women are that women have smaller kidneys with a lower glomerular filtration rate, which leads to a lower rate of drug elimination; they have a smaller liver, which leads to lower first pass drug metabolism; have a higher stomach pH; a longer gut transit time; and a higher body fat percentage.3 Furthermore, due to safety concerns, most drugs are not tested in pregnant or breastfeeding women,3,4 thus leaving a degree of uncertainty regarding whether approved drugs are indeed safe for pregnant or breastfeeding women.
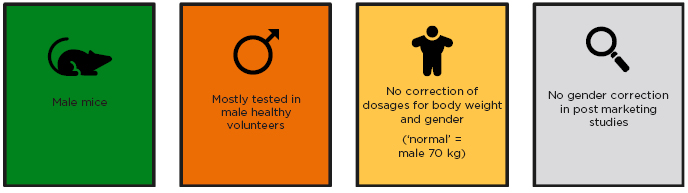
Figure 1: Drug development and sex.
Adapted from Tannenbaum et al.3
Gender differences have also been observed in male and female immunological responses to foreign and self-antigens, and these differences contribute to variations in the incidence of autoimmune diseases seen in men and women.2 Women are generally more frequently affected by autoimmune diseases than men, and this may be explained by gender differences in immunology, physiology, reproductive function, or sex hormones.2,5
axSpA and PsA are two related autoimmune diseases that are associated with elevated levels of the pro-inflammatory cytokine TNF-alpha.6 SpA comprises a group of chronic inflammatory diseases that share common pathophysiological, genetic, and clinical features, including inflammation of one or both of the sacroiliac joints (sacroiliitis). Depending on clinical manifestation, SpA can be classified as either axial or peripheral (non-axial; non-axSpA), and ankylosing spondylitis (AS) is viewed as a more advanced or severe form of axSpA.7 PsA is a heterogeneous condition that in addition to skin and nail disease (psoriasis), may manifest as arthritis (joint inflammation), enthesitis (inflammation of the sites where tendons or ligaments insert into the bone), dactylitis (inflammation of the fingers), or axial (spinal) involvement.8
A study using data pooled from four clinical trials found that, compared with men, women have a higher disease burden and less improvement in Ankylosing Spondylitis Disease Activity Score (ASDAS) after 12 weeks of TNF inhibitor treatment.9 Furthermore, women appear to have a lower response rate to TNF inhibitor treatment compared with men (1-year follow-up: women: 43%, men: 62%; 2-year follow-up: women: 46%, men: 59%).10,11 Additionally, women with axSpA also stay on the same drug for a shorter time period than men (33.4 versus 44.9 months) before discontinuing or switching treatment, mainly because of inefficacy.12
Several anti-inflammatory biologic drugs targeting TNF, such as the engineered monoclonal antibodies adalimumab, certolizumab pegol, infliximab, and golimumab, and the fusion protein etanercept, are approved for the treatment of autoimmune diseases such as axSpA and PsA.13 However, gender specific differences in the diagnosis, disease progression, and treatment options for axSpA and PsA need to be considered in order to achieve optimal disease control for both men and women.
The aim of this symposium was to highlight the unique challenges facing women diagnosed with axSpA and PsA, and to discuss how emerging data may impact on the clinical management of female axSpA and PsA patients.
Expert Discussion: Axial Spondyloarthritis
Associate Professor Helena Marzo-Ortega
axSpA is not a male specific disease. Radiographic axSpA (r-axSpA/AS) is more common in men than in women (67% versus 33%), whereas the reverse has been reported for non-radiographic axSpA (nr-axSpA) (67% in women; 33% in men).14 In nr-axSpA, in contrast to r-axSpA, substantial erosive damage to the sacroiliac joints has not yet occurred.15
Compared to males, female axSpA patients also have a lower ASAS-criteria treatment response and lower treatment improvement, more active axSpA disease, higher disease severity, and a lower quality of life.16-19 However, radiological damage and disease progression appear to be worse in men, and men are also younger at diagnosis (men: 27 years; women: 30 years; p= 0.02), and have a shorter delay to diagnosis.20,21 Although this diagnostic delay in general appears to be shrinking year-on-year, women still wait significantly longer for diagnosis than men (women: 8.8 years; men: 6.5 years; p=0.01).22,23 Interestingly, a recent report suggests that concomitant noninfectious acute anterior uveitis and chronic back pain are more common in patients with axSpA, which may help speed up the diagnosis of axSpA in both men and women.24 Pregnancy and childbirth, on the other hand, add additional complexity to the diagnosis of axSpA, as post-partum back pain may result in a false positive diagnosis of axSpA.20
Whereas men meet modified New York disease activity criteria more often, women with early axSpA have greater subjective disease activity, and tend to have more widespread pain, which may contribute to the delay to diagnosis. Additionally, women with definite sacroiliitis may experience more fatigue, peripheral involvement, and have a more relevant family history than men, and may also have more functional impairment, despite less radiological damage.20,25-27
Being affected by a potentially serious health problem may have a significant impact on mental wellbeing, and the process from initial diagnosis to acceptance of a disease, its symptoms, and treatment options has been described as a form of grieving process. During the pre-diagnosis phase, when symptoms are first recognised, patients may react with shock, denial, and frustration. In the time period following the diagnosis, low mood, and depression may slowly be replaced by engagement with the diagnosis and the disease and learning how to adjust to the new reality of living with a disease diagnosis. Once the patient comes to terms with this new situation, a new equilibrium is established.28
axSpA has a recognised negative impact on mental wellbeing, which may manifest as depression and anxiety. The mental health impact of axSpA appears to be correlated with disease activity, and seems to affect men and women equally.29-32 Patients with both r-axSpA and nr-axSpA seem to experience similarly reduced work productivity, and a study investigating work disability among male r-axSpA patients showed that almost half (45%) of patients switched to a less physically demanding job, and a quarter (24%) retired early at a mean age of 36 years.33,34 Nevertheless, non-biologic and biologic treatments are available and, in this respect, a British registry study and meta-analysis found that there is consistent evidence that treatment with biological therapy, compared with non-biological treatment regimens, significantly improves work productivity and activity impairment in people with axSpA.35
The C-axSpAnd trial, which investigated the effect of the addition of the anti-TNF biologic certolizumab pegol to non-biologic background medication, found that adding certolizumab pegol to non-biologic background medication is superior to adding placebo in patients with active nr-axSpA.36 Interestingly, a recent post-hoc analysis of disease outcomes in C-axSpAnd trial patients, stratified by symptom duration, found that patients with shorter symptom duration showed greater improvements in signs and symptoms of nr-axSpA.37
Pregnancy is an important topic for women, and rheumatologists need to bring up the subject and have a frank conversation with women diagnosed with axSpA about disease control, which drugs are compatible with pregnancy and the post-partum period, and what will happen during delivery. There are risks for adverse pregnancy outcomes in axSpA, and disease activity does matter.38 No ameliorating effect on axSpA disease activity has been reported because of pregnancy, and 60–80% of pregnant patients become symptomatic again, with increased pain and morning stiffness starting approximately at Week 20.39-41 Furthermore, active disease (ASDAS-C-reactive protein >2.1) has been reported in 78% of axSpA patients during pregnancy, most commonly in the second trimester.38 Women with axSpA also seem to have increased risk for gestational diabetes, pre-eclampsia, infection, preterm premature rupture of the membranes, small for gestational age infants, and preterm delivery.38 Active disease is a predictor of preterm delivery, and more preterm births have been reported in women with axSpA compared with population controls, especially in women not exposed to any medications.42
Both biologic and non-biologic anti-inflammatory drugs are used in axSpA, but not all drug types are appropriate during pregnancy. Nonsteroidal anti-inflammatory drugs (NSAID) may be used in pregnancy, but COX-2 selective NSAID are not recommended.43 COX-1/2 enzymes are involved in ovulation and implantation, and COX-1/2 inhibitor NSAID, with the exception of paracetamol, may be associated with an increased risk of miscarriage.44 Furthermore, NSAID are not recommended in the third trimester because of an increased risk of patent ductus arteriosus closure failure in the infant.45
A major concern with the use of biologics in pregnancy is placental transfer from the mother to the fetus. Maternal antibodies are typically transferred across the placenta to the fetal circulation through a mechanism known as transcytosis, and involves binding of the antibody Fc domain to Fc receptors situated on the surface of syncytiotrophoblast cells of the placenta.46 This mechanistic dependency on the presence of an antibody Fc domain determines how effectively antibodies are transferred across the placenta from the mother to the fetus, and may be important for how pregnant women are treated with anti-inflammatory biologics. The biologics adalimumab, etanercept, golimumab, infliximab, ixekizumab, secukinumab, and ustekinumab all contain antibody Fc domains, and adalimumab, etanercept, golimumab, and infliximab are known to cross the placenta.47-54 No data is available on transplacental transport of ixekizumab, secukinumab, and ustekinumab.55-61 Certolizumab pegol, on the other hand, is a PEGylated Fab’ fragment of a humanised anti-TNF monoclonal antibody that does not contain an Fc domain.62 As a consequence, the prospective pharmacokinetics study CRIB demonstrated minimal-to-no placental transfer of certolizumab pegol during pregnancy. Of the 14 infants that completed the study, 13 had no quantifiable levels of certolizumab pegol at birth (<0.032 μg/mL), and 1 infant had a minimal certolizumab pegol level (0.042 μg/mL; infant/mother plasma ratio: 0.09%).63 The European Medicines Agency’s (EMA) product labels state that certolizumab pegol should only be used during pregnancy if clinically needed, and that adalimumab, etanercept, and infliximab should only be used during pregnancy if clearly needed. Golimumab, ixekizumab, secukinumab, and ustekinumab are not recommended for use during pregnancy.55-63
The biologics adalimumab, certolizumab pegol, and etanercept are excreted into breastmilk, whereas no data is available for infliximab, golimumab, ixekizumab, secukinumab, or ustekinumab.55-63 The prospective pharmacokinetic study CRADLE demonstrated that the relative infant dose of certolizumab pegol transferred from plasma to breast milk is 0.15% of the maternal dose. To put these results in context, a relative infant certolizumab pegol dose below 10.00% of the maternal dose is considered unlikely to be of clinical concern, which supports continuation of certolizumab pegol treatment during breastfeeding.64 EMA label Information concludes that certolizumab pegol and adalimumab can be used during breastfeeding, whereas etanercept, golimumab, infliximab, ixekizumab, secukinumab, and ustekinumab are not recommended.55-63
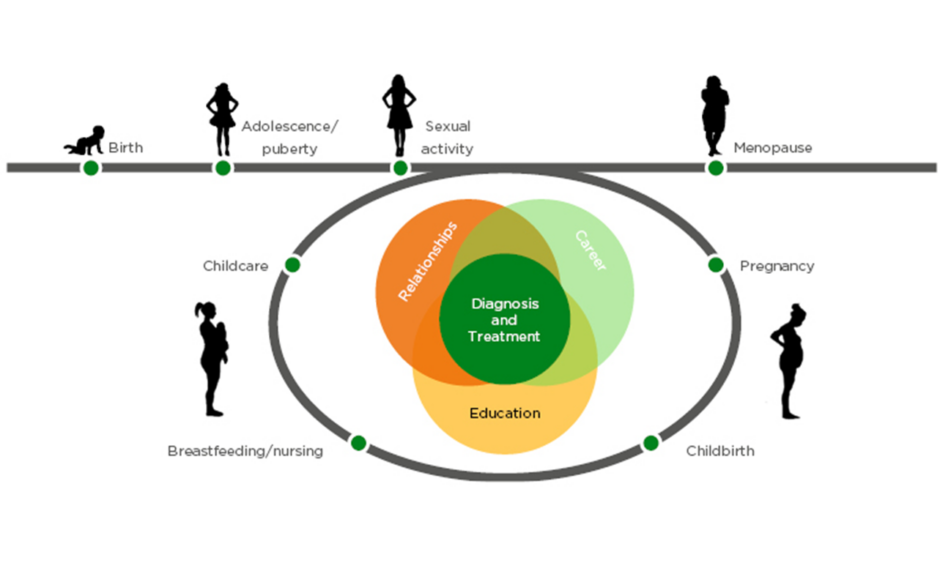
Patients and physicians often have different perspectives for the management of axSpA. Physicians may emphasise outcome measures, treatment options, and disease progression, whereas patients may put more emphasis on impact on work, friends, and family. Rheumatologists therefore need to see beyond clinical signs and recognise that different people have different needs, and that effective patient-physician communication is needed in order to optimise therapy.65 A woman’s life journey is not linear, and may be interrupted by multiple cycles of pregnancy, childbirth, breastfeeding, and childcare, which may require therapy realignment at each stage (Figure 2).66
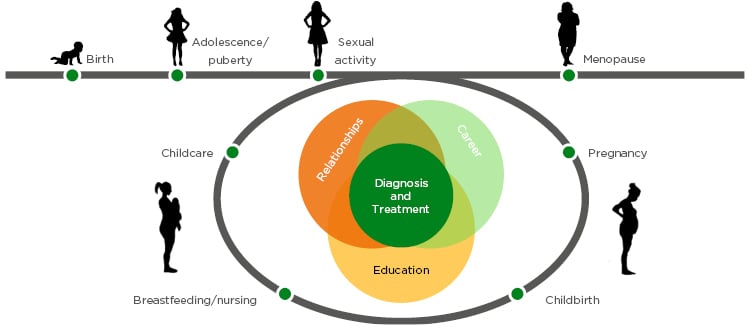
Figure 2: A woman’s life journey is not linear, and may be interrupted by multiple cycles of pregnancy, childbirth, breastfeeding, and childcare, which may require therapy realignment at each stage.
To illustrate an individual SpA patient’s life journey, the story of Hannah, an AS patient, was presented to the symposium audience. At the age of 18, Hannah first presented with back pain and, based on sacroiliac joint fusions on X-rays, was diagnosed with AS only 5 years later. In her 20s, Hannah began using a walking stick, and found that even going out for dinner was a challenge. As Hannah experienced more pain in her hands and knees, she was eventually put on a biologic which dramatically changed her life for the better. Hannah’s case related how she stopped treatment when pregnant, and how challenging disease management and motherhood can be. Hannah’s case also illustrated the lack of understanding of her work environment and the importance of getting the whole care team, including her obstetrician and midwife, involved with the treatment plan.
Expert Discussion: Psoriatic Arthritis
Doctor Laura Coates
PsA symptoms overlap with both psoriasis and rheumatoid arthritis (RA), and although joint inflammation is present in both RA and PsA, it takes longer to diagnose PsA (28.6 weeks) compared with RA (21.6 weeks).67 Women typically present with a polyarticular joint pattern and a higher number of tender and swollen joints than men at diagnosis of PsA, whereas psoriasis and pustulosis palmoplantaris is seen more frequently in men.68 Patient-reported outcomes and quality of life outcomes are worse in women than in men diagnosed with PsA, and women appear to be more disabled in daily activities and have higher disability scores.69 Furthermore, women also have different pain perception compared with men, and report a higher fatigue severity score.61 A Dutch study found that women with early PsA presented with higher SF-36 mental component and physical component summaries compared to a reference population.70 Additionally, women with PsA exhibit higher impact of disease in multiple domains, including pain, skin, fatigue, work, function, discomfort, sleep, anxiety, coping, embarrassment, participation, and depression.71 In 2015, depressive and anxiety disorders were reported in 4.4% and 3.6%, respectively, in the general population.72 In contrast, anxiety, depression, or both, has been reported by 37.0%, 22.0%, and 18.0% of patients with PsA, respectively, and depression in PsA appears to be more common in women (29.0%) than in men (19.0%).73
A treat-to-target approach, which aims to reach the target of remission or, alternatively, minimal or low disease activity, through regular monitoring and appropriate adjustment of therapy, is endorsed by both EULAR and GRAPPA guidelines for the management of PsA.74,75 MDA, defined as meeting five out of seven set criteria,76 is generally achieved by biologic therapies in approximately 40.0% of PsA patients after 1 year of therapy.77 In the TICOPA study, which evaluated tight disease control versus standard care in early PsA, fewer women achieved MDA than men in response to either standard care (men: 22.0%; women: 12.0%) or tight control (men: 35.0%; women: 23.0%) (Coates et al., unpublished data), with men outperforming women at all seven MDA domains (Figure 3).
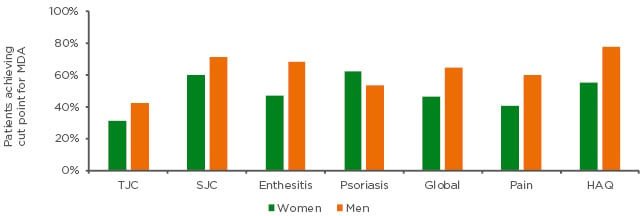
Figure 3: Women with psoriatic arthritis show lower minimal disease activity than men in the TICOPA study.
HAQ: health assessment questionnaire; MDA: minimal disease activity; SJC: swollen joint count; TJC: tender joint count
Adapted from unpublished data, Laura Coates.
Changes have also been observed in PsA disease activity, particularly for tender joint counts and C-reactive protein levels, which are elevated in women compared with men at both baseline and after 5 years of follow-up. Additionally, women have a less favourable response to therapy compared with men, lower rates of MDA (women: 33.0%; men: 50.0%), and remission (women: 13.0%; men: 25.0%) after 5 years of follow-up, and require a longer time to achieve MDA from diagnosis .68,78
Women of childbearing age with PsA face many hurdles around pregnancy and often delay or abandon decisions to start a family and to breastfeed their infants.79 Key reasons are due to misconceptions regarding their ability to conceive and carry a baby to term, fear of passing the disease to the newborn, and a lack of information and physician support.80,81 Women of childbearing age with a psoriatic disease such as PsA require adequate treatment, but despite the availability of effective therapies, their use is suboptimal in this population.79
Insight into an individual PsA patient’s experience was provided through the experience of Sophie, who was diagnosed with PsA 7–8 years after initially presenting to primary care with knee monoarthritis. When upon diagnosis she was started on methotrexate, she was told by her consultant that she would not be able to have any more children. Sophie’s psoriasis and arthritis improved during treatment, and on her own she started researching treatment options for PsA patients that are compatible with pregnancy. Together with her physician, Sophie decided on the most suitable treatment option for her considering her current priorities. As such, she is reassured that if she wants another child, she can consult with her physician and come up with a joint treatment plan that works for her and that will support her through both pregnancy and breastfeeding.
Conclusion
axSpA and PsA affect men and women differently. Compared with men, women experience longer delay to diagnosis, lower treatment response and shorter drug survival, experience more pain, carry a larger mental health burden, and experience a reduced quality of life. Women also face unique challenges associated with finding suitable anti-inflammatory treatment options that are compatible with pregnancy and breastfeeding, stressing the need for appropriate physician-patient communication and joint decision making.