Speakers: Xenofon Baraliakos,1 Christopher Ritchlin,2 Daniella Schwartz,3 Lianne Gensler,4 Jessica Walsh5,6
1. Rheumazentrum Ruhrgebiet Heme, Ruhr-University Bochum, Bochum, Germany
2. University of Rochester Medical Center, Rochester, New York, USA
3. National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA
4. University of California, San Francisco, California, USA
5. Salt Lake City Veteran Affairs, Salt Lake City, Utah, USA
6. University of Utah Medical Centers, Salt Lake City, Utah, USA
Disclosure:Dr Baraliakos received grant/research support, speaker/honoraria, or consulting fees from AbbVie, Bristol Myers Squibb, Celgene, Galapagos, Gilead, Chugai, MSD, Novartis, Pfizer, and UCB. Dr Ritchlin has recieved grant support from AbbVie and consultancy fees for AbbVie, Janssen, UCB, Sanofi, Pfizer, Novartis, and Eli Lilly. Dr Gensler has received advisor and/or consultancy honoraria from AbbVie, Eli Lilly, Gilead, GSK, Novartis, and UCB; and investigator and/or research grants from Pfizer and UCB. Dr Walsh has received grants, research support, and/or consultancy fees from Pfizer, AbbVie, Merck, Amgen, Eli Lilly, Novartis, and UCB. Dr Schwartz declared no conflicts of interest.
Acknowledgements: Medical writing assistance was provided by Dr Allison Kirsop, Scientific Writers Ltd., Edinburgh, UK. Our thanks are given to the presenters of the sessions summarised in this article.
Support: This review was funded by a medical grant from Pfizer, who had no input into its content.
Citation: EMJ Rheumatol. 2021;8[Suppl 1]:2-10.
Meeting Summary
Psoriatic arthritis (PsA) is an inflammatory condition of the joints and the areas where tendons and ligaments connect with bone. The disease affects individuals with psoriasis and many patients live with undiagnosed PsA. Earlier diagnosis is needed to allow patients to be treated with more relevant and aggressive therapies to improve quality of life. Advanced imaging techniques, such as MRI, are now being used to identify structural changes associated with this inflammatory disease, addressing the challenge of recognising separate and distinct disorders not always evident in clinical practice. The increasing technological advances being made are also utilised for qualitative assessment, with rising numbers of patient-reported outcome (PRO) instruments under development for use in the randomised trials environment, as well as clinical practice. Together, these advancements can aid early diagnosis and assist clinicians in establishing the correct treatment strategy for patients with PsA.Introduction
Psoriasis is a chronic systemic inflammatory disease affecting around 1–3% of the global population.1 The disease, which causes erythematosus and scaly skin plaques, is still poorly understood, and up to 30% of patients with psoriasis go on to further develop the seronegative spondyloarthropathy defined as PsA, a progressive joint disease characterised by inflammation and deterioration of the peripheral joints, spine, or entheses.2 PsA is also known to increase the disease burden associated with psoriasis that results in reduced patient quality of life, while increasing cardiovascular risk and healthcare costs.3 However, approximately one-half of all patients with PsA may also show axial manifestations of spondylitis and sacroiliitis, adding to both the disease burden and the diagnostic challenge. It has been suggested that approximately 15% of patients with psoriasis seen by dermatologists may have undiagnosed PsA.4
Psoriasis and PsA have some shared, but also distinct, pathogenic mechanisms which activate inflammatory pathways and drive both disorders; these include immune dysfunction and genetic and environmental factors.2 Environmental factors, such as infection, tobacco, certain drugs, and skin trauma, can promote psoriasis. Presenting patterns include five subtypes of psoriatic rash: guttate, inverse, pustular, erythrodermic, and plaque, the latter of which accounts for 85–90% of patients.5 However, diagnosis of psoriasis is largely dependent upon pattern recognition from the morphologic evaluation of characteristic skin lesions and joints.6 Spondyloarthritis (SpA) occurs when PsA involves the spine, but this separate and distinctive disorder is not always clearly identified in clinical practice, despite the different classification criteria from PsA.7
This article is a summary of presentations from the virtual American College of Rheumatology (ACR) Convergence 2020 meeting which collectively described psoriasis, PsA, and SpA, and delved into the role of immune function to help clinicians better recognise and treat these complex disorders whose reach goes beyond the skin barrier.
Cytokine Targets in Psoriatic Arthritis: The IL-23/IL-17 Axis
Doctor Christopher Ritchlin
Dr Ritchlin discussed the genetic basis of PsA, bringing insights from preclinical models that contribute towards understanding how PsA affects humans. IL-23 is a proinflammatory cytokine primarily released by dendritic cells in inflamed skin,8 as well as by macrophages and keratinocytes,9,10 and plays a role in the production of IL-17 from T cells. The process, known as the IL-23/IL-17 axis, involves both IL-17-dependent and IL-17-independent pathways.11 IL-23 can activate the differentiation of Th17 cells that results in the release of a variety of important cytokines such as IL-17A/F (heterodimeric cytokines) and IL-22. These cytokines can influence several other cell types such as osteoclast precursors (IL-17) or B-cell activators (IL-22). Additionally, the interaction of IL-23 with its receptor can promote neoangiogenesis; recruitment of neutrophils, mast cells, and macrophages; and infiltration of activated Th17 and Th22 cells.2 It is also important to highlight that there are cells that can release IL-17 independently of IL-23 activation such as the innate Type 3 lymphocyte cells and some natural killer cells; therefore, the complexities of the different pathways are of major consequence when considering the mechanism of therapeutic drugs.
IL-17A through to IL-17F are a family of cytokines which exist as either homo- or hetero-dimers, binding to specific receptors from which the downstream activation of p38 results, leading to the transcription of a variety of proinflammatory and anti-inflammatory molecules.12 Importantly, Th17 cells can be proinflammatory, anti-inflammatory, and regulatory, and it is the microenvironment of the Th17 cells which determines the function of those cells.
Preclinical Models Providing Insights into the Role of the IL-23 Pathway in PsA
Rodent models have shown how IL-23 might drive various pathways and cell populations.13-15 Data obtained on the role of IL-23 in the development of arthritis and bone metabolism suggest that targeting the IL-23 pathway, as is the case for rheumatoid arthritis (RA) therapy, may help to prevent the deterioration of bone through inflammatory processes. These preclinical studies are valuable for potentially showing how IL-23 might drive various pathways in cell populations, although directly translating these results to disease in humans is often highly problematic. Other murine studies adding to the existing knowledge base that help to further explain the pathophysiology and biology have shown how IL-23 regulates inflammatory arthritis pain; lymphocyte-independent pain can be driven by granulocyte-macrophage colony-stimulating factor,16 TNF, and C-C motif ligands.17 Zymosan was injected into the hind leg knee of IL23p19−/− (knockout) mice and pain was measured by recording the amount of time each rodent was nonweight-bearing on that extremity. The IL23p19-/- mice experienced significantly less pain than the wild-type mice (Figure 1).
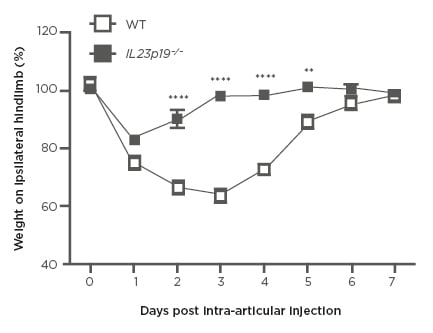
Figure 1: Development of pain-like behaviour by a change in weight distribution (incapacitance meter) in WT and IL23p19-/- mice.
WT and IL23p19−/− (knockout) mice received an intra-articular injection of zymosan. A value of <100 indicated pain.
WT: wild type.
Adapted from Lee et al.17
IL-23/IL-17 Axis in Disease Pathogenesis and the Clinical Benefits of Blockade in Psoriatic Arthritis
PsA is characterised by inflammation in the skin, bone marrow, and the gut, and the IL-23/IL-17 pathway plays a role in each of these; however, it is only now beginning to be understood how this role differs according to the tissue environment. The arrival of biologics into the clinician’s arsenal has led to improved outcomes for patients with psoriasis and PsA, but it remains true that many do not respond to treatment. To provide patients with a personalised approach to treatment management, a deeper understanding of the pathophysiology is needed along with the identification of disease progression biomarkers.2
Clinical Presentation and Comorbidities of Psoriasis and Psoriatic Arthritis
Doctor Daniella Schwartz
Dr Schwartz described the five clinical presentations of PsA that emphasise the heterogeneity of the disease: distal, oligoarticular, polyarticular, axial/spondylitis, and mutilans; distal is found alone in only 5% of patients and usually occurs in combination with other subtypes. Enthesitis can occur in 30–50% of patients, dactylitis in 40–50%, and nail dystrophy in 80–90%.18-20 There are no known biomarkers for PsA, and characteristic radiographic features reveal the deterioration of bone and cartilage along with new bone formation. Polyarthritis is the term used when five or more joints are affected and PsA should be differentiated from RA, osteoarthritis, gout, pseudogout, systemic lupus erythematosus, and other forms of SpA.
Extra-Articular Comorbidities of Patients with Psoriatic Arthritis
Associated comorbidities of PsA include uveitis, inflammatory bowel disease, metabolic syndrome, and cardiovascular disease. In patients with PsA, studies have revealed a higher increase in the risk of uveitis when compared with the general public.21-24 A strong association with Crohn’s disease, but not ulcerative colitis, was also found among these patients.21 Results from the National Health and Nutrition Examination Survey (NHANES) 2003–2006 revealed that the prevalence of metabolic syndrome in patients with psoriasis was 40% compared with 23% in the general population in the USA,25 and there is increasing evidence of inflammation as a driver of atherosclerotic cardiovascular diseases.26 Cardiovascular-related comorbidities highlighted in the literature have suggested there are shared pathogenic mechanisms with psoriasis.27,28
The question of whether a patient with psoriasis or PsA is predisposed to developing new liver disease remains unanswered as little is known of liver disease incidence in these patient populations, nor regarding any association between liver disease and skin disease severity, obesity, diabetes, and medication. A population-based study was conducted to better understand the extent of liver disease in patients with psoriasis, PsA, or RA, and the results revealed a higher risk for serious liver disease in the psoriasis and PsA groups.29 Other considerations include a higher risk of chronic kidney disease that is independent of diabetes and disease-modifying antirheumatic drug use,27 and a slightly elevated malignancy risk.30,31
Advanced Imaging and Utility of MRI in Spondyloarthritis
Doctor Xenofon Baraliakos and Doctor Lianne Gensler
Dr Baraliakos showed how imaging is a fundamental part of treatment management, and described the modalities commonly used in the diagnosis of SpA such as conventional radiography, ultrasonography, and MRI.32 Ankylosing spondylitis (AS) is one of the most common inflammatory SpA diseases and is characterised by inflammation and new bone formation, primarily in the axial skeleton. The inflammation shows a similar pattern to that of peripheral enthesitis with a severity associated with faster radiographic progression.33,34 In early inflammatory back pain, it has been shown how patients with MRI-confirmed severe sacroiliitis with human leukocyte antigen B27 positivity are more likely to develop AS in the longer-term, and this has implications for earlier diagnosis of AS and, subsequently, more aggressive therapies for patients.34 More recently, a prospective study of 21 patients with AS compared with 18 patients with degenerative disc disease investigated MRI signals of fatty lesions by immunohistological analysis of vertebral biopsies. Adipocytes were found in the bone marrow of 90.5% of patients with AS compared with 27.8% of patients with degenerative disc disease (p<0.001), and the study concluded that the presence of AS appeared to alter the bone marrow microenvironment. Higher levels of fat were associated with higher levels of new bone formation, adding to the understanding of structural progression in patients with axial SpA.
Dr Gensler presented a case study which highlighted how clinical features alone may not be enough to diagnose axial SpA. A 22-year-old male with right-sided lower back pain of 6 months’ duration had no history of anterior uveitis, inflammatory bowel disease, or psoriasis. Other than adolescent back pain and morning stiffness there did not appear to be any other factor consistent with axial SpA. As part of a study, the patient underwent several procedures beginning with X-ray, which indicated a small amount of nonspecific sclerosis on the right-hand side. A lumbar spine MRI performed prior to the study did not include the inferior portion of the sacroiliac joints and so provided no coronal oblique views, but more importantly, early axial SpA rarely shows inflammation or structural change in the spine; therefore, lumbar spine MRI was not the correct study to perform first. MRI of the sacrum/pelvis was performed without gadolinium using T1 and short-T1 Inversion Recovery (STIR) sequences to investigate structural changes including erosions and fat (T1) and bone marrow oedema (STIR). Fat, erosion, and bone marrow oedema were detected throughout the right sacroiliac joint, highlighting the importance of performing MRI on the sacrum if the radiograph is not definitive.
Treatment in Practice: Assessment Tools and Therapies for Psoriatic Arthritis
Doctor Danielle Schwartz and Doctor Jessica Walsh
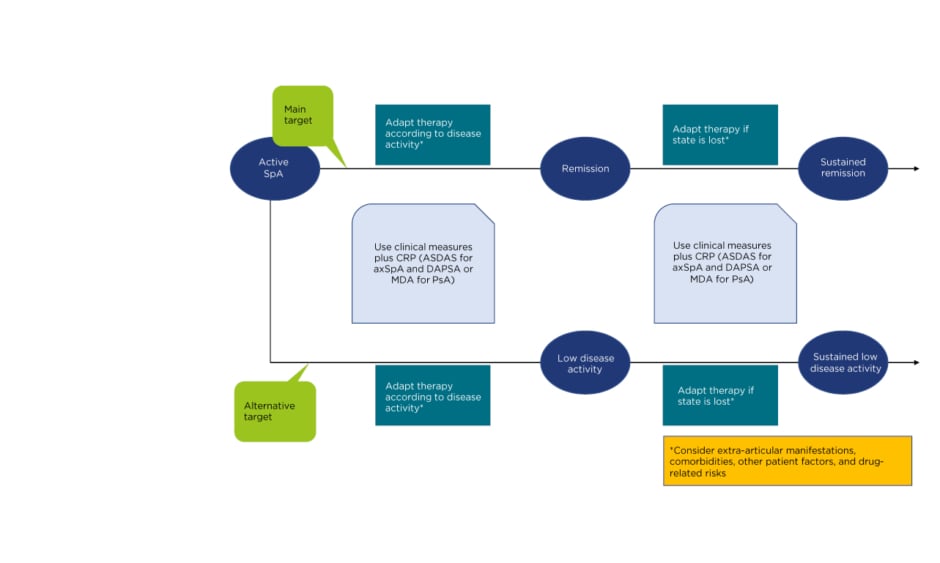
In the second part of Dr Schwartz’s presentation, the advances in treatment of PsA were discussed, including treat-to-target, early use of TNF inhibitors, and emerging drug classes. Clinicians are familiar with the strategy of treat-to-target in RA where disease activity is measured frequently, usually with the Disease Activity Score-28 (DAS-28) assessment tool; if the treatment target is not reached then treatment is modified (Figure 2).35 This management strategy should also be applied to patients with PsA; however, the challenge lies with the lack of validated disease activity measures for PsA. DAS-28 is commonly used to assess patients but can miss some important PsA-specific joints, such as the distal joints of the fingers, resulting in possible high disease activity while scoring low on DAS-28 assessment. Many PsA-specific disease activity indices have been developed, but each has its weaknesses, and none are standard of care.36 Most are comparable, and clinicians should select one, assess frequently, and adjust treatment as needed. Examples include the Disease Activity Index for Psoriatic Arthritis (DAPSA), which uses the larger 68-joint count instead of the 28-joint count but does not assess enthesitis, and the state of minimal disease activity, which does capture enthesitis but may miss patients who are moving from severe to moderate disease.
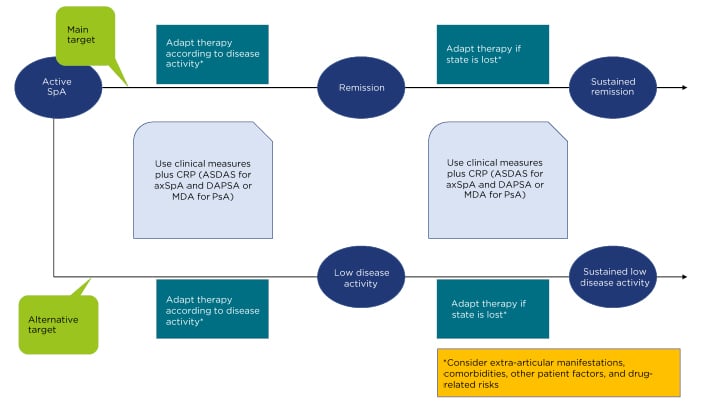
Figure 2: Algorithm based on the 2016 update of the treat-to-target recommendations for spondyloarthritis.
axSpA: axial spondyloarthritis; CRP: C-reactive protein; DAPSA: Disease Activity Index for Psoriatic Arthritis; MDA: minimal disease activity; PsA: psoriatic arthritis; SpA: spondyloarthritis.
Adapted from Smolen et al., 2018.35
Telemedicine can also play a role in PsA management and has been used successfully in multiple centres to safely manage patients. Biologics have been deemed safe to use during the coronavirus disease (COVID-19) pandemic, and although approximately 10% of patients need to be seen on a face-to-face basis, most can be safely managed using photographs, phone calls, or video calls.
Dr Walsh discussed how using PRO as an assessment tool was first used in rheumatology in the early 1980s. Since then, an expanding body of PRO literature has emerged that supports their reliability and, among other valuable insights, their ability to predict mortality and morbidity.37,38
With a shift towards bringing the patient to the centre of decision-making, PRO instruments have helped clinicians to think differently about disease assessment by broadening the concept of disease.39 However, to successfully transition PRO into clinical practice for PsA, there is a need for more real-world data. Many PRO tools are designed and tested within randomised controlled trial populations that may not be representative of patients in routine practice and where trial eligibility criteria may influence individuals’ responses to certain PRO questionnaires. How patients respond to a PRO is important to consider and contextual factors are influential in this regard. As well as the overall comorbidity burden, specific comorbidities are associated with less favourable PRO responses which include fibromyalgia, depression, smoking, alcohol use, anxiety, metabolic syndrome, neuropathic pain, osteoporosis, and restless leg syndrome. These contextual factors also extend beyond comorbidities to include demographics, healthcare settings, and caregiver burden, amongst others,40,41 and all need to be identified if PRO tools are to be applied successfully to individuals. There is a need for clinical guidance on how to interpret PRO in subsets of patients where specific contextual factors may influence responses.
Patient-Reported Outcomes Measurement Information System in Psoriatic Arthritis
As discussed by Dr Schwartz, there are many PsA-specific disease activity indices which have been developed. The Patient-Reported Outcomes Measurement Information System (PROMIS) initiative supported by the National Institutes of Health (NIH) aims to address the impracticality of using a different set of PRO for a large number of diseases. PROMIS aims to build and validate accessible item banks for key health concepts applicable to chronic diseases, for efficient and interpretable clinical practice and clinical trial applications. Benefits of PROMIS measures are that they are widely accessible, population-specific (extensive testing in over 20,000 USA patients), and use computer-adapted testing which decreases the survey-burden on patients. Rapid PRO scoring and assistance with interpretation in real-time has also enabled clinicians to use PRO in effective decision-making.42
A major drawback of PROMIS is the lack of data demonstrating that it is as good as disease-specific PRO in PsA, in all relevant domains. In addition, there is the burden of administering PRO tools. Systems need to be designed to assign a relevant PRO to each patient, and at appropriate times. They need to be able to disseminate PRO and collect responses in a secure manner, as well as facilitating access, interpretation, and response to results with minimal disruption to workflow. Regardless of the challenges involved, technology now exists to promote better understanding of the patients’ perspective and real experiences related to their illness which was previously not possible.42
Novel Treatments for Psoriatic Arthritis
Treatment for PsA is a rapidly evolving field with many new biologics: the most recent drug, guselkumab, received U.S. Food and Drug Administration (FDA) approval in 2020 (Table 1).43 Driving the latest update of the ACR guidelines43 is the understanding that oral small molecule agents generate a mild-to-moderate response in the skin and joints, whereas most biologics produce a moderate-to-excellent response in the skin and a very good response in the joints.
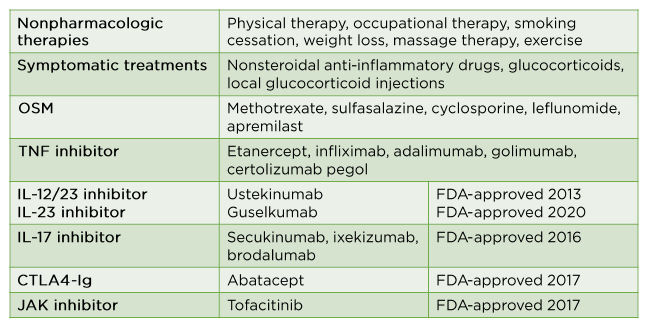
Table 1: Pharmacological, nonpharmacological, and symptomatic treatments in psoriatic arthritis.
FDA: U.S. Food and Drug Administration; OSM: oncostatin M.
Adapted from Singh et al., 2019.43
TNF and IL-17A inhibitors are established therapies in axial SpA/AS; however, contrary to expectations, clinical trials of IL-23 inhibitors failed in axial SPA, despite strong genetic and preclinical evidence and previous success in the treatment of PsA and inflammatory bowel disease. A potential explanation for this finding is that IL-23 might be more important in the initiation of disease, potentially in extra-spinal sites such as the gut. In clinical trials, JAK inhibitors have shown promising results in patients with AS. Phase II results for tofacitinib showed a clear signal for improvement of sacroiliac joint inflammation and in the spine, with a good dose–response relationship between tofacitinib and an observed improvement on follow-up MRI.44 The success of this programme resulted in additional trials of filgotinib45 (Phase II) and upadacitinib46 (Phase II/III) which demonstrated a clear clinical benefit and improvement of MRI inflammation. However, there is uncertainty concerning the cytokine signalling pathways that the JAK inhibitors engage with; once known, these may be future therapeutic targets.
Early Real-World Experience of Tofacitinib for Psoriatic Arthritis
Efficacy and safety of tofacitinib were demonstrated in Phase III trial results in patients with active PsA and an inadequate response to conventional synthetic disease-modifying antirheumatic drugs or TNF-inhibitor therapy.47,48 An analysis of USA-based claims data indicated that patients newly initiated tofacitinib treatment an average of 2 years after PsA diagnosis, and more than 60% received tofacitinib as monotherapy. As PsA and RA have similar clinical and pathological features, a sensitivity check was performed where a subcohort of patients with PsA who also had a diagnosis of RA were excluded, to reduce the chance of any misdiagnosis. High levels of persistence and adherence to tofacitinib were observed 6 months after treatment initiation.
Conclusion
PsA is common, under-recognised, and highly prevalent in patients with psoriasis. The complexities of the different pathways associated with the IL-23/IL-17 axis are highly significant for targeted therapies, and preclinical studies have provided insight into how PsA affects humans. PsA has characteristic radiographic features which need to be distinguished from other forms of arthritis with similar presentations. In axial SpA, clinical features may not be enough to make an accurate diagnosis and advanced imaging techniques are necessary to obtain detailed information relating to structural changes. Health information technology supports PRO use and can be used to assign PRO sets relevant to each patient based on diagnosis or other conditions. Systems can be designed which offer help to patients in completing PRO and to detect missing data as well as generating reminders for nonresponders or incomplete responses. The patients’ perspective is central to the diagnosis and management of PsA and technological advances in imaging, along with the development of PRO tools, contribute to earlier diagnosis and appropriate treatment for patients with PsA. The success of JAK inhibitors in axial SpA/AS has suggested there may be an alternative cytokine that is important within the inflamed axial sites which could be targeted with biologics.