Meeting Summary
Although there is no universally approved definition of moderate psoriatic arthritis (PsA), many clinicians see patients who they feel fit into this category: patients with limited joint involvement, but who might also show other manifestations of the disease, as well as a range of comorbidities. In his presentation, Dr Siebert described the challenges faced in treating this group of patients, who are mostly not captured in clinical trials. Recent advances in PsA treatment have focussed towards the severe end of the spectrum, suggesting that more must be learned around treatment options for patients with moderate disease. This represents a large unmet need. Given the heterogeneity of this patient population, a range of effective treatments is needed. Prof Gladman then presented data from longitudinal cohorts to illustrate the high burden of disease in patients with PsA who had a limited number of affected joints. By comparing patients with oligoarticular PsA (i.e., ≤4 affected joints) with those with polyarticular arthritis (≥5 affected joints), Prof Gladman showed that disease burden is not solely driven by the number of affected joints, but also by other PsA manifestations and/or comorbidities. There are clear gaps in our knowledge of PsA; to address these, population studies and trials of potential treatments are needed. Phosphodiesterase-4 (PDE4) inhibition is one potential treatment strategy that is currently being investigated. Dr Behrens described a post-hoc analysis of data pooled from three Phase III clinical trials that suggests the PDE4 inhibitor apremilast may be an effective treatment for patients with moderate PsA. It is hoped that this will be confirmed by the ongoing FOREMOST trial, a Phase IV study of apremilast in patients with oligoarticular PsA.
Introduction: Moderate Psoriatic Arthritis – What Does it Mean?
Doctor Rubén Queiro-Silva
Dr Queiro-Silva opened the session by proposing a definition of the moderate-severity PsA patient: patients associated with a limited number of affected joints, including some with oligoarthritis and some in the lower range of polyarthritis.1-3 Patients may have other core manifestations of PsA, such as dactylitis (inflammation of fingers and toes), enthesitis (inflammation of the insertion points of tendons and ligaments into bone), spinal disease, and skin and nail involvement.1-3 They may also have common comorbidities associated with PsA.4 Patients with moderate PsA therefore have a significant disease burden.
Baseline data from the Spanish Rheumatology Society’s (SER) recent-onset PsA registry confirm that patients with a low number of affected joints (i.e., who show an oligoarticular disease pattern) have pain and global disease activity scores that cannot be neglected.3
Dr Queiro-Silva illustrated the potential complexity of the moderate-severity PsA patient by describing a case from his clinic. A 24-year-old female presented with a very painful knee. An MRI scan showed that she had enthesitis and physical examination revealed dactylitis, which was not particularly painful. At the time of presentation, there was no joint synovitis.
The challenge for clinicians is therefore not only in defining the patient’s disease, but in determining the best therapy based on a number of factors, including disease severity.
Challenges in Psoriatic Arthritis Clinical Practice: Tailoring Treatments to Patients’ Profiles
Doctor Stefan Siebert
Increased knowledge regarding the immunopathogenesis of both psoriasis and PsA has led to major advances in our understanding of psoriatic disease. In the skin and enthesis, the immunological pathways are well-defined and relatively straightforward; however, in the joints, the picture is more complicated (Figure 1).5-7 This should be a consideration when a patient presents in clinic. This increased understanding of psoriatic disease has led to the development of a number of therapies directed at both extracellular and intracellular signalling pathways. The challenge for clinicians is deciding which treatment to choose for individual patients presenting with moderate disease.
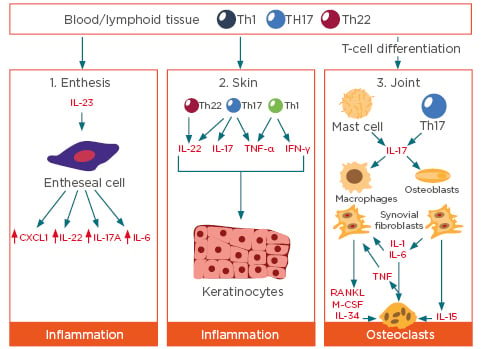
Figure 1: Inflammatory pathways in the enthesis, skin, and joints.
CXCL: chemokine ligand 1; IFN: interferon; IL: interleukin; M-CSF: macrophage colony stimulating factor; RANKL: receptor activator of nuclear factor κΒ ligand; Th: T helper; TNF: tumour necrosis factor. Adapted from Lubberts,5 Merola et al.,6 and Saxena et al.⁷
Informing Treatment Decisions in Psoriatic Arthritis
To help inform their decision, clinicians can draw on information from several sources, such as clinical trial data, guidelines and recommendations, and real-world data. However, it is important to recognise the limitations of some of these sources. For example, data from randomised clinical trials (RCT) in PsA are not easily translated into clinical practice. These trials are carried out under highly controlled conditions in homogeneous populations, meaning that they ultimately do not provide information on how to treat patients with significant comorbidities, infectious disease, or cancer. The primary endpoint in these studies is the American College of Rheumatology 20% improvement criteria (ACR20). This score has limitations in measuring improvements in patients with a limited number of affected joints, because of the obvious difficulty in showing a 20% improvement in this setting. In addition, ACR20 does not take into account the other manifestations of PsA (i.e., dactylitis, enthesitis, spinal disease, skin involvement) or quality of life (QoL). These are the issues that patients generally want to discuss in clinic. Another limitation of RCT is the over-representation of patients with more severe disease.
The European League Against Rheumatism (EULAR) has published treatment recommendations for patients with PsA.8 These recommendations move patients through a series of steps depending on how well they respond to treatment. Closer examination of the EULAR recommendations, focussing on the peripheral joints, reveals that there are fewer treatment options (with less supporting evidence) for patients with moderate PsA than for those with severe disease.8
As stated by EULAR, “The primary goal of treating patients with PsA is to maximise health-related quality of life.” Therefore, when considering which treatment to use, the principle of maximising health-related QoL should be applied.8 Low disease activity levels are associated with better health-related QoL, patient-reported outcomes, and patient-acceptable symptom state.9-11 Nevertheless, patients with residual and moderate disease activity remain undertreated: a survey of PsA patients in North America and Europe showed that approximately one-third of those who were not receiving treatment considered themselves to have moderate disease.12 This undertreatment has an impact on QoL: data from the Dutch early PsA cohort (DEPAR) showed that patients with residual disease activity despite continuous methotrexate monotherapy had worse health-related QoL scores after 6 months than those who had achieved minimal disease activity.13
Stratification of patients according to various predictive factors (biomarkers, phenotype, and comorbidities) to inform treatment choice is widely used in other fields, particularly oncology.14,15 However, in PsA, stratification according to biomarkers is not possible, as none have yet been identified. Stratification by phenotype poses challenges in terms of variation in presentation (which is heterogenous and overlapping), joint distribution (approximately one-third of patients have oligoarthritis16,17 and are less likely to achieve ACR20 after 48 weeks of treatment than those with polyarthritis),18 and disease severity (patients with moderate disease activity who do not quite fit into the current treatment guidelines).
Comorbidities are common in PsA, and the concept of multimorbidity (the presence of ≥2 long-term conditions) is an important consideration. Multimorbidity increases with age19 and is associated with reduced QoL, higher mortality, reduced socioeconomic status, higher treatment burden, higher rates of adverse drug events, and increased use of health services.19,20 Data from the Australian Rheumatology Association (ARA) database show that 58% of patients with PsA have at least two conditions other than their PsA.21 Patients with a higher number of comorbidities have worse disease activity and are less likely to respond to, or remain on, treatment.22
The prevalence of metabolic conditions, such as metabolic syndrome, obesity, and diabetes is increased in patients with PsA.23-28 In addition, patients with PsA are twice as likely to develop nonalcoholic fatty liver disease than patients with psoriasis (who in turn are twice as likely to develop this condition compared with the general population).29
Finally, patient preference should also be taken into account. A survey of patients in North America and Europe revealed several burdens that patients associate with their medication, including the need for frequent blood level monitoring, lifestyle compromises, and fear of injections.30
Summary
Although there have been major advances in PsA treatment, this has predominantly focussed on the severe end of the disease spectrum. Patients with moderate disease represent a considerable unmet need; their heterogeneity means that several treatment options are needed and tailoring treatment to the individual patient remains a challenge.
Oligoarticular Psoriatic Arthritis: Lessons from Longitudinal Cohorts
Professor Dafna Gladman
A significant barrier to the treatment of oligoarticular PsA is the requirement by some insurance payers that patients have a minimal number of affected joints to be eligible for certain treatments. However, as described below, patients with oligoarticular PsA (≤4 affected joints) have a significant burden of disease that is similar to that in patients with polyarticular disease.
Oligoarthritis is not uncommon: in a series of nine studies carried out between 1976 and 2018, each with >100 PsA patients, 25–50% of patients had oligoarthritis.31-39 Oligoarthritis occurs earlier in the disease course than polyarthritis;2 therefore, it is important that these patients are treated effectively before further joint involvement occurs.
The Burden of Oligoarticular Psoriatic Arthritis
Data from several longitudinal cohorts show that patients with oligoarticular PsA have a significant disease burden. Using data from the German Collaborative Arthritis Centres, Huscher et al.4 compared the burden of disease for patients with oligoarticular PsA (n=287) and polyarticular PsA (n=324).4 They found that 63.3% of patients with oligoarthritis had osteoproliferations, compared with 43.2% of those with polyarthritis. Comorbid conditions were also more common in oligoarticular patients: present in 82.8% of oligoarticular patients compared with 68.3% of polyarthritic patients. The study also showed that few patients who had had oligoarticular disease for <5 years were given biologics. Patients who had oligoarticular disease for ≥5 years were more likely to receive opioids than those with early oligoarticular disease or those with polyarticular disease.
Baseline data from the SER recent-onset PsA registry show that 81.5% of patients have peripheral arthritis.3 Oligoarticular PsA has traditionally been thought of as a condition affecting the larger joints. However, data from the GRAPPA Composite Exercise (GRACE) study, in which 266 patients (53.0%) had oligoarthritis, showed that smaller joints are also commonly affected.40
In the Dutch early PsA cohort (DEPAR), SF-36 scores recorded at the time of diagnosis were very similar between patients with oligoarticular disease (n=151) and those with polyarticular disease (n=91), indicating a similar burden of disease, regardless of the number of joints affected.1
Further data from DEPAR show the value of minimum disease activity (a composite measure of disease activity) in guiding treatment in both oligoarticular and polyarticular PsA.41 In addition, data from the TIght COntrol of inflammation in early Psoriatic Arthritis (TICOPA) trial show that other composite scores such as the Psoriatic Arthritis Disease Activity Score, GRAPPA composite score, and Composite Psoriatic Disease Activity Index tools were able to distinguish effectively between patients with oligoarthritis who were aggressively treated and those who received standard of care, and are therefore suitable for use even in patients with few affected joints.42
Knowledge Gaps in Oligoarticular Psoriatic Arthritis
There is clearly a large disease burden associated with oligoarticular PsA, but to be able to develop treatment strategies for these patients, a number of gaps in our knowledge must be filled. Firstly, we need to determine how to identify and measure active disease. As explained by Dr Siebert, ACR20 is not appropriate because it is not possible to show a 20% improvement when only four joints are affected. Appropriate treatment goals need to be defined, and we need to determine which treatments have efficacy in this patient group. There is an obvious need for more data in oligoarticular PsA, for example, studies looking at treatment goals that incorporate the patients’ perspective of disease burden, population studies looking at phenotype-specific outcomes, and RCT.
The FOREMOST study is one RCT that is currently underway.43 It is designed to investigate the efficacy, safety, and tolerability of the PDE4 inhibitor apremilast in patients with early, oligoarticular PsA. Patients are eligible if they have had oligoarticular PsA for <2 years and have received nonsteroidal anti-inflammatories and/or ≤1 conventional synthetic disease modifying antirheumatic drug at a stable dose for ≥3 months. Patients are randomised to apremilast or placebo and can continue to take their background therapies. Treatment will last for up to 24 weeks, after which patients can enter an extension phase and continue to receive apremilast for a further 24 weeks. The primary endpoint is minimal disease activity assessed by MDA-joints (i.e., the two articular minimal disease activity criteria plus three of the other five criteria) at Week 24.
Summary
Patients with oligoarticular disease may present with additional PsA core manifestations. In addition, these patients can also suffer from comorbidities. Overall, they can have a significant disease burden. Patients with PsA deserve appropriate treatment regardless of how many joints are affected.
Learnings from Phosphodiesterase-4 Inhibition: Optimising Outcomes for Moderate Psoriatic Arthritis
Doctor Frank Behrens
Oligoarthritis is part of the spectrum of moderate disease. Researchers in Switzerland have developed technology with a purely data-driven approach that involves a machine making this decision based on electronic patient records.44 The technology clusters patients into phenotypes and can differentiate between patients who have oligoarthritis and those who may be closer to a polyarticular phenotype. In the future, this may help clinicians with their treatment decisions. Even with this technology, it is obvious that patients with oligoarticular disease represent a large proportion of the patient population.
As described by Prof Gladman, oligoarticular and polyarticular PsA have a similar impact on patients’ QoL (as measured by SF-36). A possible explanation is that this might be driven by enthesitis, rather than joint count,1 which adds to the argument that in patients with moderate disease, clinicians need to look beyond joint counts.
The goal of treatment in PsA is to optimise long-term health-related QoL and social participation through control of signs and symptoms, prevention of structural damage, and normalisation or preservation of function.45 Approximately 70% of patients with oligoarticular PsA have non-erosive disease;38 however, if left untreated they might develop erosive disease. As described by Prof Gladman, composite scores such as clinical Disease Activity for Psoriatic Arthritis (cDAPSA) are valuable tools to measure treatment success.
Evidence for Phosphodiesterase-4 Inhibition as a Treatment Option
The PDE4 inhibitor apremilast is currently licensed for the treatment of active PsA in adult patients who have had an inadequate response or who have been intolerant to a prior disease-modifying antirheumatic drugs (DMARD) therapy. To investigate if patients treated with apremilast could reach treatment targets, an analysis was carried out on pooled data from patients in three RCT who received treatment for 1 year.46 Patients were grouped according to the cDAPSA category achieved at Week 52, and their mean cDAPSA trajectory was traced over 1 year from baseline. Patients who achieved treatment targets (low disease activity or remission) at Week 52 presented with lower disease activity at baseline. In addition, these patients had a greater and more robust decrease in cDAPSA score over 1 year than those with higher baseline disease activity (Figure 2). Probability estimates of shifting across various cDAPSA score categories from baseline to Week 52 were also presented. The probability of patients with moderate disease activity at baseline achieving treatment target (low disease activity or remission) at Week 52 was 46.9%,47 while in patients with high disease activity at baseline, the probability of achieving treatment target was 24.9%.47
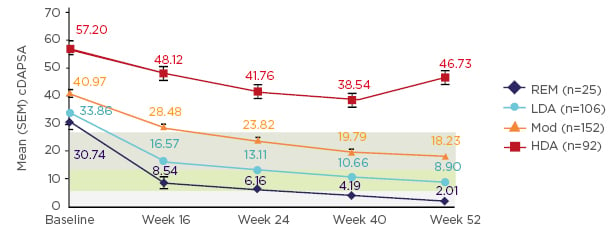
Figure 2: cDAPSA scores over 1 year of treatment with apremilast 30 mg BID, stratified by cDAPSA category at Week 52.46
High disease activity (no shading): cDAPSA >27; moderate disease activity (green shading): cDAPSA >13–≤37; low disease activity (blue shading): cDAPSA >4–≤13; remission (grey shading): cDAPSA ≤4. BID: twice-daily; cDAPSA: clinical Disease Activity for Psoriatic Arthritis; HDA: high disease activity; LDA: low disease activity; Mod: moderate disease activity; REM: remission; SEM: standard error of mean.
Control of disease activity in patients treated with apremilast also resulted in control of musculoskeletal manifestations of the disease.46 Patients who achieved treatment target had low mean swollen and tender joint counts (≤1.2 and ≤2.6, respectively) at Week 52. Mean dactylitis count was 0.0 at Week 52 in patients who achieved remission; patients who achieved low disease activity had a mean dactylitis count of 0.5. Similarly, enthesitis was also well controlled: patients who achieved remission had a mean Maastricht Ankylosing Spondylitis Enthesitis Score of 0.4 at Week 52; those with low disease activity had a mean score of 1.2.
It therefore appears that patients with moderate disease who achieve treatment target by Week 52 (based on cDAPSA score) can also achieve control of other PsA manifestations such as dactylitis and enthesitis when treated with apremilast.
In addition to RCT data, Dr Behrens also showed data from routine clinical practice in Germany. He referred to the Lapis PsA study, a prospective observational study carried out in Germany to evaluate the effectiveness of apremilast in 111 patients in a routine care setting.48 At baseline, no patients had minimal or no symptoms, as assessed using the Physician’ Global Assessment. After 4 months of treatment, this had increased to 65% of patients.
Summary
Patients with moderate PsA represent a relevant population in clinical practice. Treatment strategies need to align with individual patient disease profiles. The data presented above suggest that apremilast is a potential treatment option for patients with moderate PsA. Further evidence will become available from the randomised, controlled FOREMOST trial.
Question and Answer Session
Q: In the Swiss artificial intelligence model described by Dr Behrens, how sure can you be that the joint count is accurate?
A: Dr Behrens responded that no clear guarantee could be given, but it is also important to realise that this cannot be guaranteed in clinical and observational studies either. Prof Gladman said that there is research that shows the more highly trained an individual is, the more accurate the joint count. Dr Siebert commented that clinicians often underestimate the number of affected joints, so it is important to do a full 66/68 joint count every time the patient is in clinic.
Q: Regarding comorbidities and extra-articular involvement, which might have the greatest synergy with PsA to increase disease burden?
A: Dr Behrens responded that underestimation and undertreatment of moderate PsA leads to a cumulative disease burden. Inappropriate treatment may increase the risk for other comorbidities. Dr Siebert added that enthesitis seems to have a greater impact than other manifestations on patients’ function.
Closing Remarks
Dr Queiro-Silva asked the speakers to sum up how close they think clinicians are to being able to tailor a therapeutic approach to individual patients.
Dr Siebert reiterated that clinicians need a lot of different therapies to choose from for patients with moderate PsA. There are tools available to help clinicians; patients with moderate disease should not be allowed to drift.
Prof Gladman said that clinicians are already trying to use a personalised approach, but that it would be nice to have a biomarker or an algorithm that would help clinicians define that patient’s treatment pathway. This could happen in the next 5–10 years.
Dr Behrens stated that there have been many disappointments with biomarkers in other diseases, such as rheumatoid arthritis, and that is it unlikely that researchers will identify just a single biomarker that will give a clear picture. There are a lot of technologies being used, such as genomics and proteomics, but the information obtained has not been integrated. Artificial intelligence may provide a way forward with this. Dr Behrens estimated that we are 5–10 years away from completing the research, but perhaps 20 years away from having something tangible that can help clinicians in daily practice.
Support: The EULAR symposium and this review article have been supported and funded by Celgene.




 (apremilast) in the UK.
(apremilast) in the UK.





