Meeting Summary
The symposium at the European League Against Rheumatism (EULAR) 2017 congress aimed to provide insights into the burden of psoriatic arthritis (PsA) on patients’ daily lives, including the significant impact of unresolved musculoskeletal symptoms, and explore the current guidelines for treatment, with a view to identifying strategies to optimise disease management. Case studies were used to evaluate current strategies in PsA management and highlight the critical role of the rheumatologist in patient care. The presentations emphasised that, while patient and clinician priorities for the management of PsA may differ, wider reporting of patient perspectives in studies and patient education may aid in aligning priorities and ensuring the best quality of life (QoL) for patients. The importance of tailoring treatment to the individual was reinforced, and the need to take into account all aspects of disease, including comorbidities and patient relevant outcomes, highlighted.
UNDERSTANDING PSORIATIC ARTHRITIS FROM THE PATIENT’S PERSPECTIVE
The Psoriatic Arthritis Patient Journey: From Diagnosis Through to Clinical Management
Doctor Juan Gomez-Reino
PsA is a progressive disease that is often preceded by dermatological symptoms, with 72% of patients developing skin lesions prior to joint disease.1 In some cases, skin manifestations can appear ≤10 years before a diagnosis of PsA is made, with patients commonly presenting initially to a primary care physician before being seen by a dermatologist. Joint symptoms, such as pain, stiffness (in 60% of patients), dactylitis (45%), and enthesitis (31%), are often not recognised as related to the skin symptoms by the physician or the patient, and referral to a rheumatologist and diagnosis of PsA is often delayed. There is no distinctive pattern of skin and joint symptoms, and the absence of a biomarker for PsA also contributes to the delay in diagnosis; ≤50% of patients with both psoriasis (PsO) and PsA remain undiagnosed.2 Speed of diagnosis is dependent on the primary care physician or dermatologist having the necessary understanding of the condition to promptly refer to a rheumatologist for assessment.3 Without appropriate treatment, 58% of patients will have erosions in more than five joints after 10 years,4 impacting both QoL and function. Management of patients with PsA is made more challenging by the development of certain comorbidities requiring additional treatments, which may also restrict the therapeutic options for controlling PsA.1,3
Burden of Psoriatic Arthritis on the Patient’s Quality of Life
Doctor Ana-Maria Orbai
The challenges faced by patients with PsA are diverse and include physical, psychological, social, and economic factors.5 Pain associated with the disease is a critical issue for patients with PsA,1 with fatigue,6 emotional distress, and depression also contributing significantly to disease burden.7
Chronic rheumatic diseases have an increased inflammatory burden that is associated with the development of comorbidities including obesity, hypertension, metabolic syndrome, and infection, with 42% of patients experiencing three or more comorbid conditions.8 Depression or anxiety occur in approximately one in four patients.9 Multiple studies have shown that patients with PsA have a decreased QoL compared with patients with PsO alone.10 There is also an interplay between factors influencing QoL, with emotional aspects impacting on physical and social function.8 Depression and anxiety impair the ability of patients to self-manage and cope with PsA, leading to a cycle of lack of sleep, low energy levels, and withdrawal from personal relationships.3
PsA impacts on multiple aspects of a patient’s daily life; patients with worse musculoskeletal symptoms or musculoskeletal damage from the disease have difficulty with many daily tasks. In a recent survey, >20% of patients reported struggling or being unable to bend down to pick up clothing from the floor, and approximately 15% reported difficulty or inability to walk outside on flat ground (either ‘unable to do’ or ‘with much difficulty’).1
How Can we Improve Alignment with Our Patients?
Professor Laure Gossec
Being able to continue with daily activities is of critical importance to patients and is also a key consideration for physicians when setting goals of treatment. As inflammation is typically driving joint damage in patients and the development of comorbidities, physicians commonly focus on targeting this aspect with drug therapy, with the goal to maintain function and normal daily activities.11 For patients, improving QoL is their primary goal.7,12
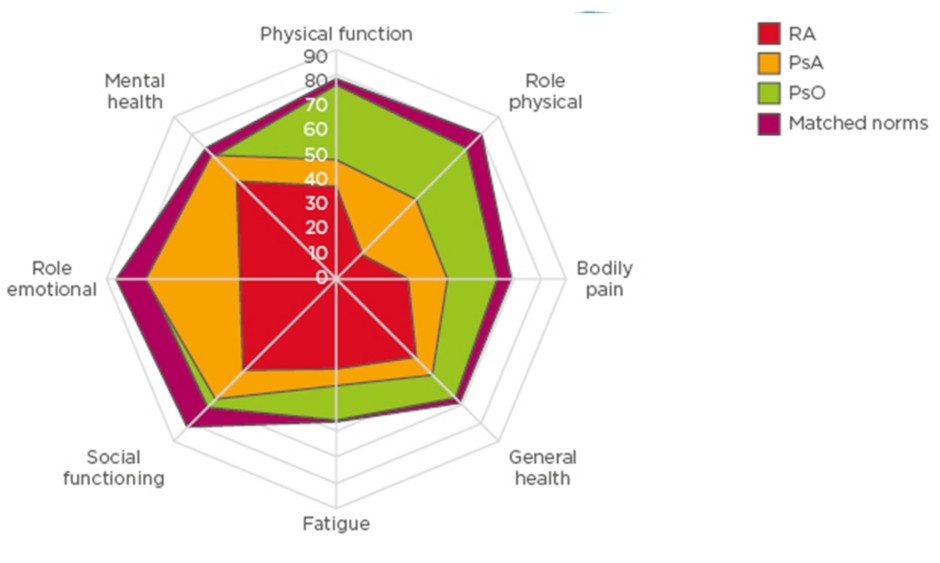
A comparison of the impact of disease on QoL using the 36-item short form health survey (SF-36) across rheumatoid arthritis (RA), PsO, and PsA indicates that there is a greater impact on bodily pain, general health, and physical function in patients with PsA and RA, than in PsO (Figure 1).12 A systemic literature review of 11 studies highlighted the wide range of symptoms experienced by patients with PsA and ranked their importance.13 Pain was the highest priority physical symptom for patients, with social interaction the highest priority social symptom. Their findings were reinforced in a survey, in which pain was ranked as the highest priority by 84% of respondents.11
Figure 1 Comparison of health-related quality of life in rheumatoid arthritis, psoriatic arthritis,
and psoriasis.
PsA psoriatic arthritis; PsO psoriasis; RA rheumatoid arthritis.
Adapted from Strand et al.12
In order to achieve a more patient-centric approach to management, greater use of patient activity scores and numerical rating scales should be used to evaluate not only pain, but also other factors of importance to patients, such as fatigue. Such tools include the health assessment questionnaire (HAQ)14 and the PsA impact of disease (PsAID) questionnaire,15 which is available from the EULAR website in 40 languages. Greater use of such tools in recent years has improved the evaluation of a wider range of factors important to patients.16,17
Patient education may help individuals to more clearly communicate about their specific concerns and expectations to healthcare professionals. Recommendations have been published by EULAR, which strongly recommend patient education forms an integral part of patient care and should be tailored and needs based, as well as presented in a variety of formats.18 Educational resources are available to aid clinicians with this, including written information, self-management programmes, and resources to aid the sharing of experiences between patients.18
OPTIMISING PSORIATIC ARTHRITIS CARE IN CLINICAL PRACTICE
Integrating the Patient Perspective: Best Practices in Psoriatic Arthritis
Professor Laure Gossec
Two sets of guidelines for the treatment of PsA were published in 2016: the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) guidelines19 and the EULAR recommendations.20
GRAPPA recommendations use the initial symptom, such as peripheral arthritis, enthesitis, or skin or nail disease, to determine treatment approach, although they do not include a specific treatment target.19 Recommendations state that patients with peripheral joint involvement may be treated with either non-steroidal anti-inflammatory drugs (NSAIDs), with or without glucocorticoid injections, a conventional synthetic disease-modifying anti-rheumatic drug (DMARD), a tumour necrosis factor (TNF) inhibitor, or a phosphodiesterase-4 (PDE-4) inhibitor. As a second step, TNF inhibitors, interleukin (IL)-12/23, IL-17, or PDE-4 inhibitors can be considered.19
The EULAR recommendations state that the main treatment target should be clinical remission, or low disease activity where remission is not achievable, based on both patient and physician considerations.20 NSAIDs and/or local glucocorticoid injections are recommended for peripheral joint involvement, followed by a conventional synthetic DMARD, preferentially methotrexate. Subsequent treatment depends on the presence of adverse prognostic factors, including number of joints involved, acute-phase reactants, and previous joint damage. Patients without adverse prognostic factors can begin a second conventional synthetic DMARD, while patients with such factors may be recommended TNF, IL-12/23, or IL-17 inhibitors.
Treat-to-target is well established in RA, but less well studied in PsA. However, the recent randomised controlled TICOPA trial in patients with PsA revealed that such an approach resulted in significant improvements in both clinical and patient-reported outcomes.20,21
The perception of remission differs between patient and physician. The EULAR definition is stable remission with no acute-phase reactants, namely disease activity, pain, patient global function, QoL, fatigue, and systemic inflammation.22,23 Physicians will commonly base remission status on either the EULAR principle of no active joints and normal acute phase reactants, but scores can also be used such as the Disease Activity Score (DAS28), though it is not optimal in PsA, the Disease Activity of Psoriatic Arthritis (DAPSA) score,24 or minimal disease activity (MDA). Patients, on the other hand, are more focussed on the resolution of symptoms, return to pre-disease normality, and other patient-related outcomes. This disparity between patient and physician perception of remission requires alignment, and a combination of endpoints that are important to both parties should be considered.25
The Rheumatologist at the Centre of Comprehensive Care
Doctor Juan Gomez-Reino
The heterogeneous nature of PsA makes it a challenging condition to diagnose and treat. Currently, the primary outcome required for US Food and Drug Administration (FDA) approval of new PsA therapies is the American College of Rheumatology 20 criteria (ACR20), which assesses disease activity in the joints.23 However, ACR20 does not include the assessment of other domains known to affect patients with PsA, including enthesitis, dactylitis, axial involvement, skin disease, nail disease, or peripheral arthritis.23,25,26
Composite outcomes for disease assessment, which integrate a number of patient and clinician measures, have been developed to allow a more comprehensive evaluation of treatment response.25 Current rheumatological practice estimates show that 82% of clinicians include joint counts when assessing the disease, but as few as 14% employ a treat-to-target approach to patient management.19 Implementing treat-to-target in practice requires a common target to be determined by both the patient and clinician, which also ensures that all PsA domains are assessed using validated methods. To date, MDA criteria have been suggested to be the best compromise between comprehensive assessments and clinical feasibility. Additionally, this assessment should take no more than 5–10 minutes to complete, further simplifying its integration into routine clinical practice.19,27
Comorbidities in patients with PsA are also an important consideration in the management of this condition. Beyond management of joint disease, rheumatologists need to become more active in addressing the wider aspects of PsA. EULAR recommendations reinforce the importance of the rheumatologist in taking on the comprehensive comorbidity management of the patient, highlighting the need for participation in systematic and routine assessment, data collection, and treatment.
Maximising Communication Among Stakeholders
Doctor Ana-Maria Orbai
A multidisciplinary approach is essential to ensuring comprehensive care to patients with PsA, with rheumatologists ideally positioned to facilitate collaborative care and communication with general practitioners, patient advocacy groups, dermatologists, psychologists, and other specialists critical to the design and delivery of an effective treatment strategy.
A recent systematic review of collaborative care (N=3 studies) demonstrated improvements in skin and joint symptoms in 56–82% of patients after treatment changes under dermatology–rheumatology management. Up to 94% of patients were reported to be ‘very satisfied’ with multidisciplinary care.28 Patients’ satisfaction ratings were 4.9 out of 5 for collaborative consultation, compared with 2.9 out of 5 for separate, routine consultations.
Both EULAR and GRAPPA recommend shared treatment decision-making between the patient and physician, with consideration of efficacy, safety, and costs, as well as patient preferences and expectations.3,19,20 Furthermore, patients should be encouraged to take a more active role in their care, including self-management of pain, involving training by qualified personnel to aid both management and assessment, and to regularly feedback to their rheumatologist. To facilitate this process, channels of communication between the patient and physician should always remain open.3 Patients involved in the decision-making process appear to have improved outcomes and higher satisfaction with their healthcare.29
Effective communication is key in optimising the management and care of patients with PsA. There are several suggested methods for improving and/or maintaining effective patient-physician communication, which include providing education on the disease, its progression, and its long-term consequences; being supportive of patients recording their symptoms and communicating these to clinicians for appropriate and efficient referral; providing small amounts of information regularly over time, in combination with goal setting and action planning during disease progression; and empowering patients to feel comfortable requesting information about their condition at any point in time.3
A fundamental barrier to improving patient–physician communication is a declining number of rheumatologists. Based on recent trends, the number of rheumatologists is predicted to decline by 30% between 2015 and 2030.30 Innovative approaches are needed to ensure access to care.
Greater use of technology may help overcome challenges associated with reduced access to rheumatologists; however, tools to facilitate efficient disease management are currently underutilised, and there is no effective resource to aid patient awareness and self-management of their condition.31,32 Electronic data capture, which would allow individuals to input patient-reported outcomes into a digital tool prior to visits, can improve communication through completion of patient-reported outcomes prior to the clinic visit and at the same time facilitate a treat-to-target approach. Using the same tool, rheumatologists would input physical examination findings during the visit.19 Specialist software can then be used to calculate composite targets based on patient and physician-reported outcomes and determine disease activity status; and also suggest follow-up times based on data history.19
An example of the potential value that technology can bring to shared care is exemplified by a smartphone application for RA, based on a DAS28 predictive model consisting of subjective measures and a performance measure (Figure 2). The application was used by nine patients with RA to record patient-reported outcomes over a 3-month period and register their physical activity using their smartphone accelerometer. DAS28 scores predicted by the calculator were compared with DAS28 calculated by a physician. A good correlation was found between the physician and application-calculated DAS28, and indicates that such a model shows promise for integrating routine self-assessments of rheumatologic disease in clinical practice.
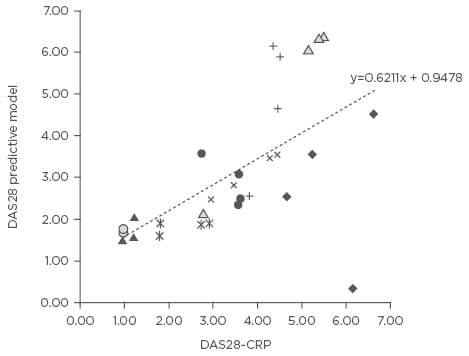
Figure 2: Self-assessed disease activity measured via smartphone correlated well with patient’s actual disease.
DAS28: Disease Activity Score 28; CRP: C-reactive protein.
Adapted from Nishiguchi et al.33
RISING TO THE CHALLENGE: PATIENT CASE STUDIES
Case 1: Managing a Patient with Refractory Joint Issues Despite Resolution of Other Symptoms
Doctor Ana-Maria Orbai
Prior to a PsA diagnosis, patients will often experience joint pain, stiffness, and fatigue.34 As many as 47% of patients can continue to experience erosive disease, despite clinical improvement, and 65% of patients with power Doppler ultrasound synovitis at baseline will experience a flare compared with patients without.35,36
Patient Case 1
Patient file: Jeremy is a white, 28-year-old male, with a 5-year history of PsA, and a positive family history of the disease. His BMI is 32 kg/m2, with a blood pressure of 150/90 mmHg. In addition to PsA, Jeremy’s comorbidities include hypercholesterolaemia and hypertension, which are managed through diet and exercise. Jeremy’s skin symptoms are currently well managed but pain/stiffness in the hand and ankle joints persist. Jeremy also experiences 1–2 flares a year where the metacarpophalangeal, proximal interphalangeal joints, ankle, and knee joints become tender and swollen. Jeremy prefers to cope with these flare ups on his own.Initial Treatment Response and Assessment Approaches
Jeremy was started and maintained on a conventional synthetic DMARD (csDMARD) for 18 months before being switched to a TNF inhibitor. Despite 3.5 years of anti-TNF therapy, he has seven swollen joints, nine tender joints, and a HAQ disability score of 1.5, none of which met the MDA criteria cut-offs. Jeremy’s clinical DAPSA score, a clinical score used to determine disease activity state based on tender and swollen joint counts, patient global assessment, and a pain scale, was 18.5, which categorises Jeremy in moderate disease activity state. Jeremy’s PsAID score was 4.15, which is slightly above the patient-acceptable symptom state (cut-off of ≤4).37
Considerations for Case Study 1
Jeremy’s active psoriatic disease combined with under-reporting of flare symptoms can make management of the disease using a treat-to-target approach especially challenging. Data collection of 66/68 joint counts, which include upper and lower extremity joints, use of ultrasound imaging data if needed, radiographs of the joints to screen for damage and inflammatory markers are recommended in the management of this specific patient. If axial disease is suspected, a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) is recommended. An open discussion of the findings and their implications is encouraged with the patient to help facilitate shared decision making.
Effect of Weight
Jeremy’s BMI of 32 presents a challenge in the effective treatment of his PsA. Recent studies have shown that MDA is difficult to achieve in patients with a BMI >30 compared with patients with a BMI <30. Furthermore, weight loss of >5% has been shown to increase the chances of achieving MDA in patients with PsA.38,39
Case 2: What is the Best Target for Patients with Multiple Comorbidities?
Doctor Juan Gomez-Reino
Patient Case 2
Patient file: Luis is a white, 40-year-old male diagnosed with PsO in 2012, and then PsA in 2015. His BMI is 39 with a blood pressure of 160/110 mmHg. He has a family history of PsO. Luis’s comorbidities include hypertension and coronary artery disease, obesity, hyperlipidaemia, and Type 2 diabetes mellitus. Luis admits to struggling with treatment adherence due to polypharmacy. Luis has experienced a recent worsening of pain/swelling in several digits, and PsO of the scalp. His history of symptoms includes pain, stiffness, and swelling around joints of the lower extremities and distal joints, a swollen right knee, and erythematous papulosquamous plaques over the extensor surfaces. Luis was started on local glucocorticoid injections, csDMARD, and folic acid for 6 months, then switched to csDMARD, biologic DMARD, and folic acid for 1.5 years.Patients with PsA will often suffer from many comorbidities,8 with the increased inflammatory burden commonly associated with increased comorbid conditions, including cardiovascular disease (CVD).40 Up to 20% of patients seen by rheumatologists do not have a primary care practitioner41 and EULAR recommends rheumatologists are responsible for CVD management.42 Accordingly, rheumatologists can help patients with inflammatory joint disorders, allowing them to manage and reduce the risk of CVD.43
Considerations for Case Study 2
NSAIDs can be used to control RA-specific inflammation, which can improve CVD risk.43 However, NSAIDs must be used with caution in patients with multiple comorbidities, especially for patients with documented CVD or in the presence of CVD risk factors, e.g. hypertension.42 Glucocorticoids can be used with caution, but are recommended at the lowest effective dose, for the shortest duration, and they should be tapered as rapidly as clinically feasible. Systemic glucocorticoids would not be recommended in this case. Patients such as Luis seldom reach a target of remission; therefore, it is more reasonable to set a target of MDA.
Where comorbidities exist, challenges with compliance need to be borne in mind. Failure to adhere to treatment can occur due to a variety of reasons, including medication type, number of daily doses, doubts about treatment necessity, concerns about side effects, and low satisfaction in the physician–patient relationship.44 Luis has admitted that he struggles with treatment adherence owing to multiple medications, and treatment options that reduce the number of daily doses of treatments he is taking each day should be considered. Concerns regarding side effects associated with individual therapies also need to be addressed.
Case 3: How does Patient-Physician Discordance Influence Disease Management?
Professor Laure Gossec
Patient Case 3
Patient file: Kim is a 39-year-old female with skin and joint symptoms that have been well-managed for 2.5 years. Kim discussed her experiences with other patients with PsA via an online forum and approached her doctor due to feeling very tired. Although she has been treated with csDMARDs for 2.5 years, current symptoms at assessment included fatigue, joint pain/swelling, and enthesitis. In order to reduce fatigue, a biologic was started alongside a recommendation of exercise, which may aid fatigue.Patient Priorities
Fatigue reduction is a priority for patients due to its effects on daily life (Figure 3).22 There is often misalignment between a patient’s disease assessment and their physicians due to the difference in priorities; almost one-third of patients with PsA are not aligned with their physicians regarding disease activity, with patients often believing they are doing worse than their physicians.7
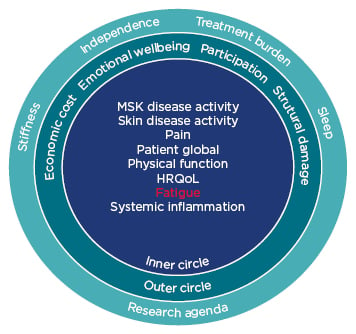
Figure 3: Updated psoriatic arthritis core domain set.
HRQoL: health-related quality of life; MSK: musculoskeletal.
Adapted from Orbai et al.22