BACKGROUND AND AIMS
Acute urinary retention (AUR) is a serious complication of progressive benign prostatic hyperplasia (BPH) that produces significant discomfort to patients. Although commonly managed with first-line medical therapy, treatment failure often leads to invasive surgery such as transurethral resection of the prostate or holmium laser enucleation of the prostate, both of which are associated with morbidity and long-term complications.1 The prostatic urethral lift (PUL) is a minimally-invasive surgical therapy that utilises small permanent implants to mechanically open the urinary channel, and has been shown to improve the quality of life (QoL) for BPH patients with minimal morbidity.2-4 Here, the authors assess safety and feasibility of PUL in subjects with AUR.
MATERIALS AND METHODS
The PULSAR study enrolled males ≥50 years of age across six UK sites with symptomatic BPH, prostate volumes ≤100 cc, and AUR with at least one failed trial without catheter while on β-blockers. Void trials, symptom response (measured by the International Prostate Symptom Score [IPSS] and Benign Prostatic Hyperplasia Impact Index [BPHII]), QoL, uroflowmetry (measured by maximum flow rate [Qmax] and postvoid residual urine volume), and urodynamic assessments (change in detrusor pressure at maximal flow rate [pdet.Qmax] and Bladder Outlet Obstruction Index [BOOI]) were examined post-PUL for 12 months. Baseline urodynamic assessment was not used for inclusion or exclusion criteria. Sexual Health Inventory for Men [SHIM] and return to normal scores were also analysed postprocedurally. Results were compared with the PUL randomised control trial (L.I.F.T. study). Rates of adverse events and catheter independence were calculated.
RESULTS
The PULSAR study population of 52 men was significantly older (71.4±7.9 years versus 67.0±8.6 years; p<0.01) and had larger prostates (55.2±22.8 cc versus 44.5±12.5 cc; p<0.0001) compared with the L.I.F.T. study. Out of the subjects, 67% failed ≥2 trials without catheter prior to PUL, and mean catheter dependence was 132 days. Presence of lower urinary tract symptoms for >6 years was experienced by 40% of the subjects. After PUL, IPSS symptom response was similar to L.I.F.T.; however, QoL was significantly better for AUR subjects throughout follow-up (Table 1), and BPHII was better for AUR subjects at 1, 3, and 6 months post-PUL. Of the subjects, 67% became catheter-free by 1 month, 77% by 3 months, and 81% by 6 months. Three subjects (5.7%) remained catheterised at the end of the study; however, all switched from an indwelling catheter to intermittent self-catheterisation. Eighty percent of subjects were able to stop treatment with β-blockers. Five subjects (9.6%) underwent surgery and two subjects (3.8%) had a repeat PUL. Twelve-month improvement in pdet.Qmax and BOOI was seen in 12 subjects (-15.0±11.7, p=0.001; -18.2±24.0, p=0.02, respectively). Baseline SHIM was stable or improved throughout follow-up, and 88% of subjects returned to normal in an average of 8.5 days. Adverse events were typically mild-to-moderate and resolved within 2 weeks.
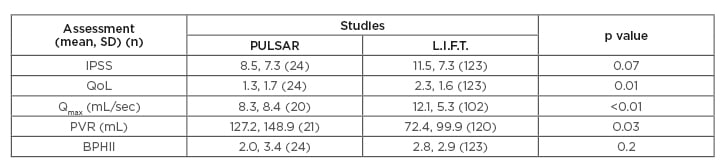
Table 1: Twelve-month outcomes of PULSAR patients compared with randomised L.I.F.T. study.
BPHII: Benign Prostatic Hyperplasia Impact Index; IPSS: International Prostate Symptom Score; L.I.F.T.: Luminal Improvement Following Prostatic Tissue; PULSAR: Prostatic Urethral Lift in Subjects with Acute Urinary Retention; PVR: postvoid residual urine volume; Qmax: maximum flow rate; QoL: quality of life; SD: standard deviation.
CONCLUSION
PUL safely and quickly restores normal voiding and improves lower urinary tract symptoms in BPH patients with AUR without the risks associated with cavitating procedures. Improved QoL scores in AUR patients indicate PUL can have a profound impact on these patients’ lives, compared to patients who are voiding prior to PUL.








