INTRODUCTION AND OBJECTIVES
The German PROBASE trial is an ongoing, randomised, risk-adapted prostate cancer (PCa) screening trial that primarily evaluates the start of prostate-specific antigen (PSA)-based screening at 45 versus 50 years.1-3 Active surveillance (AS) is a treatment option that may reduce treatment-related harms in clinically insignificant screen-detected PCa, especially in younger men.4 In this analysis, the authors present the first interim results of AS as a treatment modality within PROBASE.
MATERIALS AND METHODS
Basic descriptive statistics were used to report treatment choices and outcomes. A Kaplan–Meier time-to-event analysis was performed to analyse the rate of AS discontinuation. Since the start of the study in 2014, 364 PCa cases out of 46,495 PROBASE participants were registered at the time of data extraction (October 1, 2024). This analysis was limited to International Society of Urological Pathology (ISUP) Grade Group (GG) 1 (n=83) and 2 (n=158) PCa cases with available first-line treatment data. For the Kaplan–Meier analysis, participants who either withdrew from the study (n=2) or were lost to follow-up (n=14) after the start of AS were excluded.
RESULTS
The median age at PCa diagnosis was 50 years (interquartile range [IQR]: 47–51). A total of 91 patients started AS, including 72% of men diagnosed with ISUP GG 1 PCa (n=60). The remainder of ISUP GG 1 and 2 patients (n=150) underwent up-front radical prostatectomy (RP) or radiotherapy. Figure 1 shows the course of AS over time since the start of the AS protocol. The median time on AS was 41.2 months. In 85% (95% CI: 74.9–96.1%), 60% (95% CI: 45.3–78.8%), and 40% (95% CI: 24.2–65.1%) of men, AS delayed definitive treatment for at least 2, 3, and 4 years, respectively. So far, 19 patients have discontinued AS, with a median time to radical treatment of 24 months (IQR: 15–33; range 7–46). Importantly, of 13 patients with documented repeat biopsy prior to AS discontinuation, only seven had histological tumour upgrading (≥ISUP GG2 with >10% Gleason pattern 4) as a hard criterion for AS discontinuation.
Few of the 13 cases with available RP pathology reports had adverse pathological outcomes (R1: 3/13, pT3-4: 1/13, pN1: 0/13, ISUP GG 3–5: 0/13) at RP after AS discontinuation. After RP, 12 of 14 patients had a complete PSA response and remained free of biochemical recurrence with a median follow-up of 25.5 months (IQR: 13.5–35.4; range 5.3–66.5).
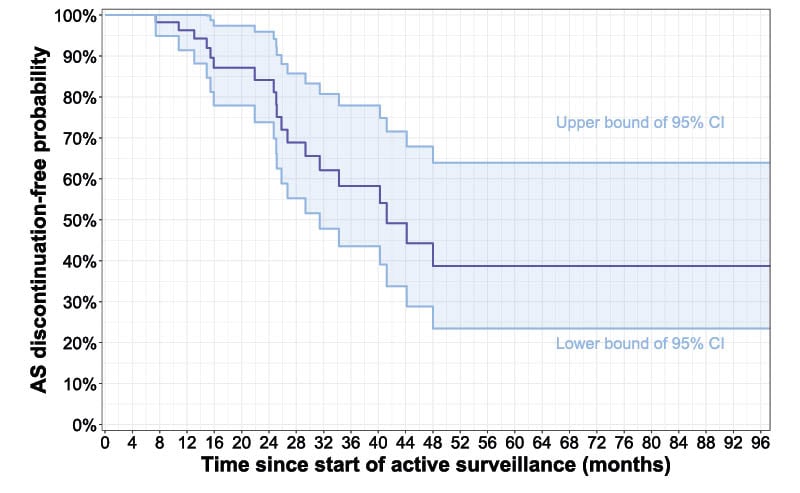
Figure 1: Course of active surveillance for International Society of Urological Pathology Grade Group 1 and 2 prostate cancer in PROBASE.
AS: active surveillance.
CONCLUSION
In PROBASE, AS deferred definitive treatment in half of the young men with low-risk and favourable intermediate-risk screen-detected PCa by nearly 3.5 years or longer, with acceptable short-term oncological outcomes after RP following AS discontinuation.