BACKGROUND AND AIMS
Antimuscarinic drugs are the first-line choice in the treatment of patients with neurogenic detrusor overactivity (NDO), even if the relative literature is still limited.1,2 Fesoterodine fumarate is the newest antimuscarinic drug. No study has been yet published about the use of fesoterodine fumarate in patients affected by neurogenic lower urinary tract dysfunction. The aim of the authors’ study was to determine the efficacy of fesoterodine fumarate for the treatment of patients with NDO due to multiple sclerosis (MS) or spinal cord lesion (SCL).
MATERIALS AND METHODS
The study was designed as an open-label prospective interventional trial without a control group. Eligible subjects for enrolment were considered patients between 18 and 80 years of age affected by NDO, confirmed by urodynamic studies (UDS), secondary to MS or SCL of at least 6 months before their enrolment. All participants provided informed consent and the study had the approval of the scientific and ethics committee of the National Rehabilitation Center, Athens, Greece. It was considered unethical to create a placebo (or a non-therapy) control group as the increased detrusor pressure might harm the unit under test of the patients. On the other hand, previous studies on other antimuscarinics proved that the placebo effect is rather ’limited’ in such a cohort of neurological patients.
A 2-week wash-out period was requested prior to enrolment for patients under drug medication for the treatment of NDO. All patients underwent a first confirmatory baseline UDS and fulfilled the SF-Qualiveen as a quality of life (QoL) questionnaire.3-5 Afterwards, all subjects received a treatment of 8 mg fesoterodine daily for 3 months. At the completion of this period, they repeated the UDS and SF-Qualiveen. Each UDS was performed with the same equipment in the same environment from the same clinician, unaware of the study hypothesis, according to the International Classification for Standards’ (ICS) urodynamic practices and terms.6 The primary endpoint of the study was the change from baseline to end of treatment in maximum detrusor pressure (Pdetmax) during the filling phase of the UDS, and whether the use of fesoterodine fumarate would decrease it. Secondary endpoints included changes from baseline to end of treatment in bladder capacity and compliance. By analysing the questionnaires pre- and post-treatment, patient QoL was evaluated. Statistical analysis was performed using SPSS® Statistics 26 (IBM, Endicott, New York, USA).
RESULTS
One hundred and thirty-seven patients were recruited and 124 of them, 68 males and 56 females, completed the study. Of the included patients, 95 had SCL (60 paraplegia and 35 tetraplegia) and 29 had MS. The urodynamic parameters Pdetmax, bladder capacity, and compliance were estimated for the whole group before and after treatment and were proved to be statistically different when compared to the baseline (p<0.001 for all variables), according to the Wilcoxon test for a non-parametric sample. Changes in urodynamics parameters were also significant in each of the paraplegic, tetraplegic and MS groups (p<0.001) (Table 1). According to all the domains of SF-Qualiveen, QoL also improved. There was a significant difference in SF-Qualiveen score after treatment with fesoterodine in all patients, independently of the diagnosis.
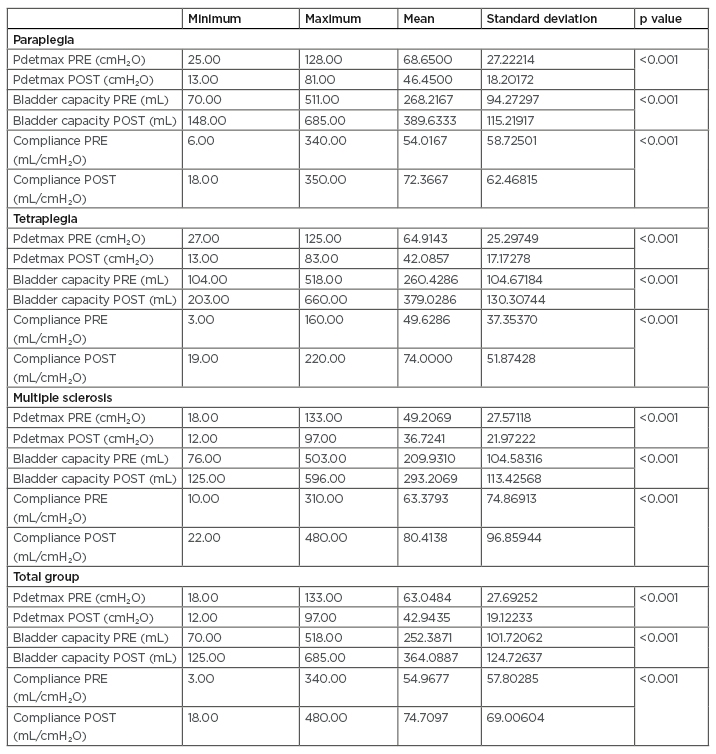
Table 1: Variance of the urodynamic variables after treatment with fesoterodine fumarate.
Pdetmax: maximum detrusor pressure; POST: after treatment; PRE: before treatment.
CONCLUSIONS
Fesoterodine fumarate is an efficacious drug in patients with SCL and MS, as it significantly decreases the detrusor pressure, increases the bladder capacity and compliance, and improves the QoL.








