INTRODUCTION AND OBJECTIVES
Active surveillance (AS) is the preferred management strategy for low-risk prostate cancer (PCa), with evidence supporting its safety for both Grade Group (GG) 11 and favourable-risk GG2 disease.2,3 However, almost half of men discontinue AS within 5 years, often without clear evidence of disease progression but for reasons such as patient anxiety.4 Here, the authors compare the reasons for discontinuation between GG1 and GG2 PCa, questioning whether AS is equally tolerated from a psychosocial perspective in these groups.
MATERIALS AND METHODS
The authors evaluated men with GG1 (n=1328) and GG2 (n=120) PCa from the global Prostate Cancer Research International Active Surveillance (PRIAS) database who had an MRI at the time of diagnosis and attended at least one follow-up visit. Cumulative incidences (CIN) of remaining on AS, discontinuation based on protocol advice, and discontinuation for other reasons (e.g., patient anxiety) were calculated using competing risk analyses. Additionally, protocol-based reasons of discontinuation between GG1 and GG2 were compared.
RESULTS
Median follow-up for men still on AS was 1.68 years (interquartile range: 0.80–3.34) in GG1 and 1.44 years (interquartile range: 0.76–2.34) in GG2. The CIN of still being on AS at 3 years post-diagnosis was similar in both groups: 0.71 (95% CI: 0.67–0.74) in GG1 versus 0.60 (95% CI: 0.49–0.73) in GG2 (Figure 1).
The CIN of discontinuation based on protocol advice was also similar: 0.19 (95% CI: 0.16–0.22) in GG1 versus 0.24 (95% CI: 0.14–0.35) in GG2. A CIN of 0.08 (95% CI: 0.06–0.10) in GG1 and 0.15 (95% CI: 0.05–0.24) in GG2 reflects discontinuation for other reasons. Among men who discontinued based on protocol advice within 3 years, biopsy upgrading was the main reason in both groups, with 85% in GG1 and 59% in GG2.
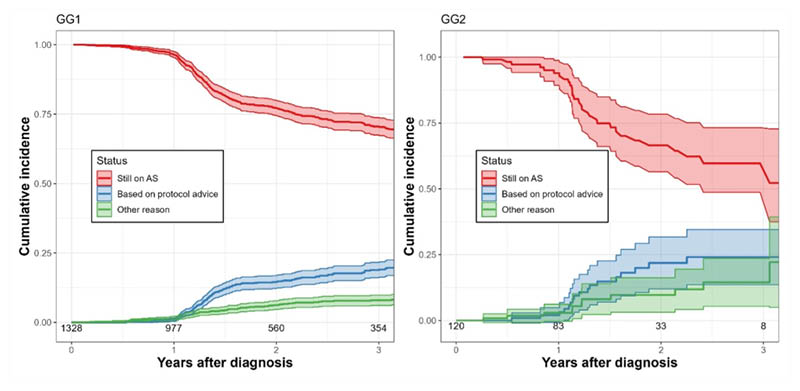
Figure 1: Cumulative incidences of active surveillance discontinuation for Grade Group 1 and Grade Group 2 prostate cancer.
AS: active surveillance; GG1: Grade Group 1; GG2: Grade Group 2.
CONCLUSION
Both the overall probability of remaining on AS at 3 years post-diagnosis and discontinuing based on protocol advice are similar for men with GG1 and GG2 PCa, with biopsy upgrading being the most common reason for discontinuation in both groups. Importantly, there seems to be no difference in the probability of discontinuation due to other reasons, such as patient anxiety, suggesting that patients and physicians tolerate AS as a management strategy for favourable GG2 PCa.