BACKGROUND AND AIMS
Patients with high-risk upper tract urothelial carcinoma (UTUC) typically undergo radical nephroureterectomy (RNU), while kidney-sparing surgery (KSS) is most commonly reserved for those in the low-risk category.1 However, comparability in terms of oncological outcomes between KSS and RNU in high-risk patients has been suggested.2,3 In this context, the specific role of a robot-assisted approach in high-risk patients has not been thoroughly investigated.
This multi-institutional retrospective cohort study aims to directly compare the oncological and functional outcomes of high-risk patients with distal ureteral tumours undergoing either robot-assisted distal ureterectomy (RADU) or robot-assisted RNU (RANU).
MATERIALS AND METHODS
Robotic Surgery for Upper Tract Urothelial Cancer Study (ROBUUST) is an international, multicentre, ongoing registry of patients who underwent RNU or KSS (segmental ureterectomy or endoscopic ablation) for UTUC at participating centres from 2015–2022. The ROBUUST database was queried to retrieve high-risk patients, according to European Association of Urology (EAU) risk stratification criteria,1 by distal ureteral tumours, who underwent RADU or RANU.
Local recurrence-free survival (RFS), distant metastasis-free survival (MFS), and overall survival (OS) were estimated using the Kaplan–Meier method with a 3-year cut-off, and log-rank test was used to assess the statistical difference between cohorts. After adjusting for potential confounders, a multivariable Cox proportional hazard model was plotted to evaluate significant predictors of each oncological outcome. Functional outcomes included serum creatinine and estimated glomerular filtration rate (eGFR) at post-operative Day 1, discharge, post-operative 3 and 12 months, and last follow-up; and eGFR variation between baseline and time of last follow-up.
RESULTS
A 3-year RFS of 8.7 months (95% confidence interval [CI]: 5–12) was observed in the RANU group, compared to 6 months for the RADU group (95% CI: 6–10), with no significant differences between the two treatment groups (p=0.5; Figure 1A). RANU patients had a 3-year MFS of 7 months (95% CI: 4–12), compared to RADU patients who achieved a 3-year MFS of 12 months (95% CI: 3–18), with no differences between the two groups (p=0.5; Figure 1B). A 3-year OS of 17 months (95% CI: 4–24) for the RADU group, and 18 months (95% CI: 9–21) for the RANU group was observed, with no difference between the two (p=0.8; Figure 1C). In the multivariate Cox proportional hazard model, surgical treatment did not emerge as a significant predictor for either RFS (hazard ratio [HR]: 0.64; 95% CI: 0.53–1.22), MFS (HR: 0.86; 95% CI: 0.66–2.60), or OS (HR: 0.86; 95% CI: 0.77–1.30). At last follow-up, patients undergoing RADU had significantly better post-operative renal function, with a significantly higher mean (±standard deviation) eGFR variation in the RANU group compared to the RADU group (p=0.01).
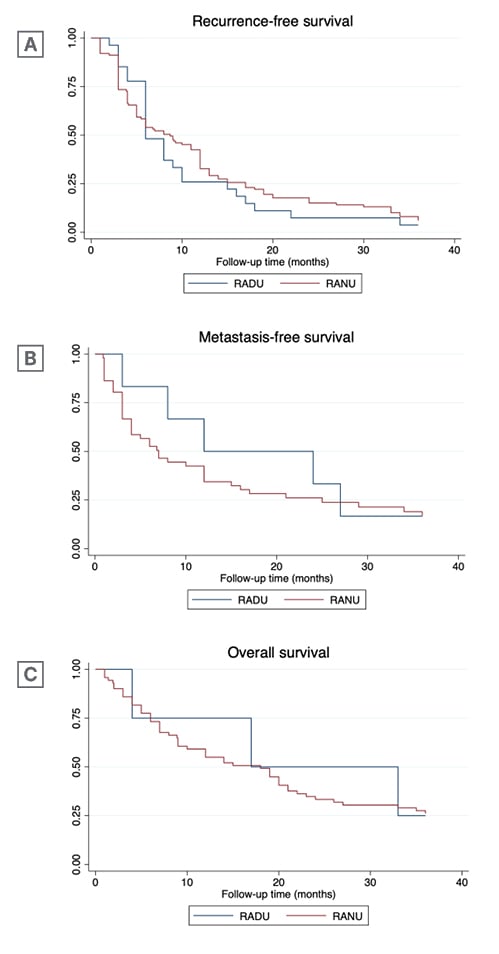
Figure 1: Kaplan–Meier analysis comparing robot-assisted distal ureterectomy and robot-assisted nephroureterectomy patients for time-to-event outcomes.
RADU: robot-assisted distal ureterectomy; RANU: robot-assisted radical nephroureterectomy.
CONCLUSION
The authors’ findings suggest comparable outcomes in terms of RFS, MFS, and OS between RADU and RANU patients, and an advantage in terms of postoperative renal function preservation. Moreover, a significantly better preservation of postoperative renal function was observed among RADU patients. Given its advantages, KSS should be considered as a potential suitable option for selected patients with high-risk distal ureteral UTUC.







