Abstract
Until recently, men diagnosed with high-risk non-metastatic castrate-resistant prostate cancer (nmCRPC) were faced with the prospect of a relatively short reprieve from symptomatic progression before the onset of metastatic disease. Crossing this red line represents a turning point in the disease, characterised by debilitating pain, greater functional and emotional impairment, a need for additional treatments, and, eventually, death. Delaying time to metastatic progression has the potential to limit symptomatic progression, reduce morbidity and mortality, and maintain quality of life in nmCRPC, and efforts have been made to establish the validity of metastatic-free survival (MFS) as a valid and meaningful clinical endpoint in this setting. The approval in Europe of apalutamide and enzalutamide based on the Phase III SPARTAN (NCT01946204) and PROSPER (NCT02003924) trials, respectively, with MFS as a primary endpoint (defined as time from randomisation to first radiographic detection of distant metastases, or death) sets a new precedent for future trials in nmCRPC. Although median overall survival (OS) has not yet been reached in either trial, meta-analyses of the two studies suggest a significant improvement in OS alongside a confirmed improvement in MFS for novel anti-androgens versus placebo. A third drug, darolutamide, has also been submitted for regulatory approval to treat nmCRPC. This review aims to summarise the evidence supporting the use of MFS as a clinical endpoint and the benefit of delaying metastasis in men with high-risk nmCRPC, and to discuss the influence of next-generation imaging on prostate cancer staging.
INTRODUCTION
Prostate cancer is the second most common cancer in men worldwide, and the third most common cause of cancer mortality in European men.1 The majority of prostate cancers are first detected during routine screening for prostate-specific antigen (PSA) or by digital rectal exam. The optimal strategy for PSA screening has been extensively debated. Current advice from the European Association of Urology (EAU) is to first screen at age 50, or age 45 in African American men or men with a family history of prostate cancer, with follow-up intervals of 2 or 8 years depending on baseline PSA.2 Most men are initially diagnosed with localised disease and treated according to their prognosis as determined by D’Amico classification. At this stage, for many men, first treatment with curative intent usually consists of radical prostatectomy and/or radiotherapy.2 Men with a rising PSA level after therapy with curative intent are said to be in ‘biochemical failure’: a disease state that indicates tumour recurrence or micrometastatic growth undetectable on imaging.3 Androgen deprivation therapy (ADT) in the adjuvant setting can suppress bone marrow micrometastases in around 80% of men in biochemical failure, thereby reducing PSA levels, but most will inevitably experience further rises as they progress to non-metastatic castrate-resistant prostate cancer (nmCRPC).4,5
Advances in our understanding of prostate cancer biology and drug development led to the first European approvals for men with nmCRPC at high risk of metastases or death: enzalutamide in October 2018 and apalutamide in January 2019.6,7 A third anti-androgen, darolutamide, was submitted for European marketing authorisation in March 2019.8 The approvals of enzalutamide and apalutamide were the first to be based on the intermediate endpoint of metastasis-free survival (MFS) rather than the conventional endpoint of overall survival (OS), setting a new precedent for future clinical trials in non-metastatic prostate cancer.9,10 The purpose of this multi-disciplinary review is to summarise the evidence supporting the use of MFS as a clinical endpoint and the benefit of delaying metastasis in men with high-risk nmCRPC. The authors will also discuss the influence of new imaging techniques on prostate cancer staging, that were not available when the Phase III trials for apalutamide, enzalutamide, and darolutamide were designed.
The Diagnosis of Metastases is a Turning Point in Prostate Cancer
The majority of all cancer-related deaths are due to metastatic progression rather than local disease, with some estimates as high as 90%.11 Bone is the predominant site of distant metastases in prostate cancer and is associated with poor survival outcomes. Median survival of patients with newly diagnosed metastases is approximately 3.5 years,2 indicating a high lethality associated with progressive morbidities that include, but are not limited to, pathologic fractures, spinal compression and other skeletal-related events (SRE), and disrupted haematopoiesis.12 SRE occur on average every 3–6 months, though often in clusters, and become more frequent with more extensive disease. Indeed, men with bone metastases live under constant threat of debilitating symptoms and spikes in bone pain, and the majority of pathological metastatic fractures never fully heal.13 Although bone is often the only distant metastatic site in men with mCRPC, prostate cancer can also spread to the lungs and liver, with reported incidence rates at autopsy of 46% and 25%, respectively. Other reported metastatic sites in patients with prostate cancer include the pleura and adrenal glands.14
The combined burden of prostate cancer and its treatments can greatly impact patients’ health-related quality of life (HRQoL), leading to profound functional and emotional impairment and negative effects on self-perception and relationships with others.15 Men with prostate cancer are up to five times more likely to experience symptoms of emotional distress than men of a similar demographic in the general population.16 As might be expected, delaying progression and initiation of chemotherapy is psychologically very important for men with nmCRPC.16-18 In the metastatic setting, emotional distress felt prior to progression is amplified.17
Defining High-Risk Non-Metastatic Castrate-Resistant Prostate Cancer
The standard definition of CRPC is based on castrate levels of testosterone and evidence of PSA progression.2 Absence of metastases should be confirmed by imaging, including bone and computed tomography (CT) scans of the chest, abdomen, and pelvis. Progression is defined by the EAU as a PSA value of >2 ng/mL and three consecutive rises in PSA level 1 week apart, with two 50% increases over nadir.2 Because the natural history of nmCRPC is relatively indolent, even in men with rising PSA despite ADT and castrate levels of testosterone, identification of men at high risk of progression can ensure that life-prolonging treatment is provided as early as possible and avoid over-imaging in those at low risk of progression.19,20
Fortunately, patients at high risk of metastases or death can be readily identified. Studies have identified short PSA doubling time (PSADT) as a key predictor of a range of survival outcomes, including OS, prostate cancer-specific mortality, time to first bone metastasis, and bone MFS.19-21 The first Phase III study to use continuous PSA kinetics to identify men at high risk of developing bone metastasis defined high-risk nmCRPC as a PSA pre-randomisation ≥8.0 ng/mL, PSADT ≤10 months at baseline, or both.21 The same threshold for PSADT has been applied in the Phase III registration trials for apalutamide (SPARTAN), enzalutamide (PROSPER), and darolutamide (ARAMIS).9,10,22
Recently, an attempt to formally define clinically relevant cut-points proposed adopting PSADT <9 months as an indicator of high risk for metastatic progression not limited to bone. Additional cut-points for very high risk (PSADT <3 months), intermediate risk (PSADT 9 to <15 months), and low risk (PSADT ≥15 months) were also suggested, consistent with validated cut-points used for risk stratification of hormone-sensitive prostate cancer. Median time to metastasis was 9 months for very-high-risk patients, 19 months for high-risk patients, 40 months for intermediate-risk patients, and 50 months for low-risk patients. The authors suggested that observation may be a valid management approach for men in the low-risk group, who accounted for almost half of the study population.20
The Need for Intermediate Endpoints in Prostate Cancer
Advances in prostate cancer diagnosis and management mean that the time from detection of rising PSA to disease progression and death can span several years. The availability of multiple sequential therapy options has transformed the prostate cancer treatment landscape, to the extent that it is increasingly difficult to associate OS with any single treatment approach. Although OS remains the gold standard endpoint in prostate cancer trials, the growing impracticalities of conducting studies on this primary endpoint (including the need for larger and longer studies to capture a meaningful number of survival outcomes, and potential for confounding due to comorbid conditions and non-cancer-related deaths) are recognised by regulatory authorities.23
Of course, this situation is not unique to prostate cancer. Intermediate clinical endpoints (ICE) have been used to inform clinical decisions and facilitate drug development in other solid tumours for almost 30 years.24 Disease-free survival (DFS) was the first surrogate for OS to be accepted as a regulatory endpoint when the U.S. Food and Drug Administration (FDA) approved tamoxifen as adjuvant therapy for node-negative breast cancer in 1990. Similarly to DFS, progression-free survival (PFS) also captures all-cause mortality, with the added advantages of requiring fewer patients and a shorter follow-up than OS. PFS allows for measurement of stable disease and does not rely on tumour shrinkage to capture the effects of anticancer treatment. Time to progression (TTP) shares a benefit of PFS over OS in that the endpoint is reached sooner. However, PFS is preferred over TTP as it does not censor deaths that occur before progression, which has the potential to introduce bias. PFS and TTP were first accepted as regulatory endpoints in 1991 and 1994, respectively, supporting the approvals of carboplatin in ovarian cancer and paclitaxel as a second-line option for women with breast cancer.24
The use of ICE in other early-stage oncology settings may offer a closer corollary with nmCRPC. Disease or recurrence-free survival is routinely used in adjuvant breast and colon cancer trials to assess metastatic risk and is accepted as a surrogate for OS. Thus, while patients are still followed for OS, demonstration of improved OS is not usually required for drug approval. Like MFS in nmCRPC, these endpoints are signposts for progression from localised disease to metastatic spread, a transition that triggers further treatment and leads to a progressive increase in symptoms, greater morbidity and mortality, increased healthcare resource utilisation, and deterioration of HRQoL.23
Several attempts have been made to identify ICE in prostate cancer. ICE evaluated in the setting of localised disease include time to biochemical failure, PSADT ≤3 months, PSA nadir, end-of-treatment PSA, DFS, and MFS.25 In 2015, the Intermediate Clinical Endpoints in Cancer of the Prostate (ICECaP) working group initiated a formal approach to assessing which of these ICE, if any, may serve as robust surrogates for OS. This collective effort enabled a direct comparison of DFS and MFS using individual patient data (IPD) pooled from 19 trials (21,140 patients), of whom approximately two-thirds had high-risk disease. Correlation with OS was greater for MFS than DFS, partly because DFS not only captures true progression but also local recurrences that may be indolent and/or cured with salvage therapy.26 PFS-based ICE were not included in the IPD analysis as they were less reliable predictors of prostate cancer-related mortality.25-27
More recently, Jackson et al.28 further validated the use of MFS in surgically treated patients undergoing postprostatectomy radiation therapy, an under-represented group in the earlier IPD analysis. A landmark analysis evaluating ICE (biochemical failure, MFS, and CRPC) identified 5-year MFS as the most robust endpoint for OS in this subset of patients.28 As a whole, the available data support the notion that prevention of early metastatic events is a strong surrogate for OS in nmCRPC regardless of whether initial treatment was radiation-based or surgical.29
Metastasis-Free Survival in Phase III Clinical Trials
Interest in MFS as a surrogate for OS in registration studies was spurred by a 2011 report of the FDA Oncologic Drugs Advisory Committee (ODAC) stating that MFS is a reasonable regulatory endpoint, provided that a substantial magnitude of improvement can be demonstrated alongside a favourable benefit-risk evaluation.30 Following this ruling, multiple studies were initiated of systemic therapies in nmCRPC with MFS as a primary endpoint and OS as a secondary endpoint. Whereas early studies did not always exclude local progression, current definitions of MFS recognise that the impact on survival of local progression events is relatively minor compared with the impact of distant metastases, and such events are necessarily excluded.23,30 In SPARTAN, PROSPER, and ARAMIS, MFS was the primary endpoint.9,10,22
Results of the primary analysis demonstrate remarkably similar median MFS across the three trials (36.6–40.5 months; all p<0.001).9,10,22 The additional MFS benefit over ADT alone was substantial (21.9–24.3 months). In SPARTAN and PROSPER, the risk of distant metastatic progression or death was reduced by 72% and 71%, respectively; the corresponding reduction in ARAMIS was 59%. Median OS, a secondary endpoint in each of the trials, had not yet been reached in any active treatment arm at the time of primary analysis due to too few events (Table 1).9,10,22 Assuming a drug class effect of these novel nonsteroidal anti-androgens, two meta-analyses of SPARTAN and PROSPER have confirmed improved MFS and demonstrated significant increases in OS versus placebo (hazard ratio: 0.76; p=0.03).31,32
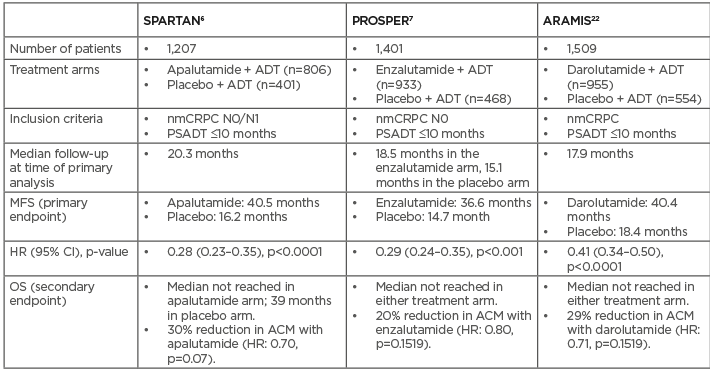
Table 1: Metastasis-free survival and overall survival in Phase III trials of next-generation androgen receptor inhibitors.
ACM: all-cause mortality; ADT: androgen-deprivation therapy; CI: confidence interval; HR: hazard ratio; MFS: metastasis-free survival; N0: absence of nodal disease; N1: Metastatic spread to local (pelvic) lymph nodes only; nmCRPC: non-metastatic castrate-resistant prostate cancer; OS: overall survival; PSADT: prostate-specific antigen doubling time.
Approval of apalutamide and enzalutamide by the European Medicines Agency (EMA) was based on the evidence that improvement in MFS offers direct benefit to the patient. Although both trial populations were enriched with patients judged to be at high risk of metastatic progression or death, large and consistent MFS benefits and a favourable safety profile were observed for both treatments (Table 2).23
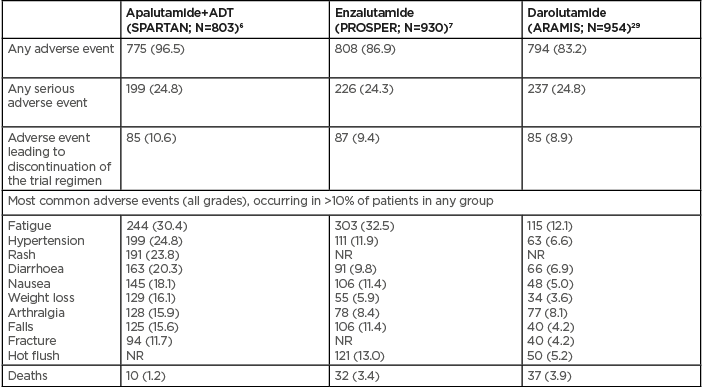
Table 2: Safety and tolerability in Phase III trials of next-generation androgen receptor inhibitors.6,7,29
NR: not reported.
Globally, regulatory acceptance of a surrogate outcome generally requires demonstration of an association of the surrogate with preferred endpoints, such as OS or validated patient-reported outcomes.33 An association between prolonged MFS and improved OS was recently shown in a real-world setting using retrospectively collected data.37 Given that OS is inherently dependent on MFS in nmCRPC, a landmark analysis of patients in SPARTAN sought to further investigate the correlation of MFS to OS using standard nonparametric tests and a more robust parametric method developed by Fleischer et al.35 that accounts for the dependence of OS on MFS and for patient censoring.36 As may be expected, patients who developed metastases after 6, 9, and 12 months of apalutamide treatment had shorter median OS compared with patients without metastases. This association remained significant (p<0.0001) after adjusting for baseline prognostic factors such as pelvic lymph node involvement, use of bone-sparing agents, PSADT (≤6 months versus >6 months), and PSA (continuous variable). Nonparametric methods identified a positive correlation between MFS and OS (Pearson and Spearman correlation coefficients of 0.66 and 0.62, respectively; p<0.0001). The Fleischer test confirmed the positive association with an estimated correlation coefficient of 0.69 and indicated that MFS could account for 48–53% of variability in OS. Median time to metastasis was 40.5 months and median post-metastasis survival was 26.1 months.37
The Value of Delaying Metastasis in Non-Metastatic Castrate-Resistant Prostate Cancer
Each of the Phase III studies in nmCRPC to date confirm the value of delaying metastasis across multiple additional efficacy and patient-reported outcomes. Results from SPARTAN confirm the association of shorter PSADT with an increased risk of metastatic progression or death in men with nmCRPC treated with ADT.9,38 PSADT <10 months was also associated with an increased risk of symptomatic progression and a shorter second progression-free survival, an exploratory endpoint defined as the time from randomisation to disease progression on subsequent anticancer therapy, or death (Figure 1). In the primary analysis, apalutamide with ongoing ADT was found to reduce the risk for second progression or death by 51% versus placebo.9 A post-hoc analysis with an additional 1 year of follow-up showed sustained improvement in second progression-free survival, suggesting that early intervention in the nmCRPC stage delays further downstream progression.39
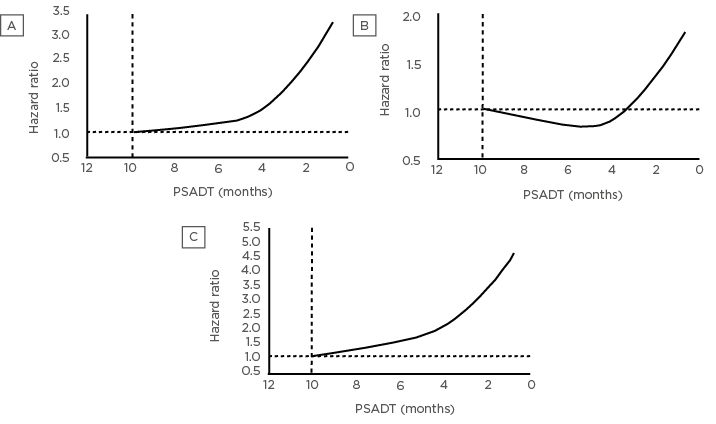
Figure 1: Non-metastatic castrate-resistant prostate cancer patients with prostate-specific antigen doubling time ≤10 months are at significant risk of (A) metastases or death, (B) symptomatic progression, and (C) secondary progression.45
Data are from the placebo arm of SPARTAN. nmCRPC: non-metastatic castrate-resistant prostate cancer; PSADT: prostate-specific antigen doubling time.
A multivariate analysis at 12 months identified apalutamide treatment, longer PSADT (>6 months versus ≤6 months), absence of locoregional disease, lower Gleason score at diagnosis (≤7 versus ≥8), and lower baseline PSA (≤7.8 ng/mL versus >7.8 ng/mL) as independent predictors of longer MFS.40 Furthermore, improvements in MFS with apalutamide versus placebo, each with ongoing ADT, were confirmed across a range of clinically relevant subgroups (age <75 years, baseline Eastern Cooperative Oncology Group performance status of 0, Gleason score ≥8 at diagnosis, ≥2 prior hormonal therapies, locoregional disease, and no baseline use of bone-sparing agents), and for all baseline PSA and PSADT values.40 Apalutamide with ongoing ADT was also associated with longer time to initiation of cytotoxic chemotherapy versus placebo.9
Given the emotional impact of rising PSA levels in men with nmCRPC and the symptomatic and HRQoL burden on patients with progressive disease, prolonging times to PSA progression, symptomatic progression, and deterioration of HRQoL are important considerations from the patient perspective. In SPARTAN, risk of symptomatic progression or death was reduced by 55% and risk of PSA progression by 94% with apalutamide with ongoing ADT compared with placebo with ongoing ADT.9 Median time to symptomatic or PSA progression was not reached in either treatment arm. Consistent with the longer time to symptomatic progression, deterioration in HRQoL over 12 months was delayed in patients treated with apalutamide with ongoing ADT based on group mean Functional Assessment of Cancer Therapy-Prostate (FACT-P) total and subscale sores, and FACT-General total score. That a new treatment for nmCRPC is able to maintain HRQoL while significantly prolonging MFS and symptomatic PFS represents an important advance in disease management.39
The key secondary efficacy endpoints in PROSPER were time to PSA progression and time to first use of a subsequent antineoplastic therapy. Enzalutamide reduced the risk of PSA progression by 93% and the risk of first subsequent antineoplastic therapy by 79% compared with placebo, with a median follow-up of 18.5 months and 15.1 months, respectively.10 Patient-reported outcomes included FACT-P in addition to the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-PR25), EuroQoL 5D visual analogue scale (EQ-VAS), and clinically meaningful pain progression assessed by the Brief Pain Inventory Short Form (BPI-SF) pain severity score. A time to deterioration analysis showed statistically significant differences in favour of enzalutamide versus placebo for the FACT-P total score and prostate cancer and emotional wellbeing subscales using a confirmed-at-next-visit analysis.41 Enzalutamide also significantly prolonged time to clinically meaningful pain progression (hazard ratio: 0.75; p=0.028), time to clinically meaningful symptom worsening on the urinary and bowel symptom subscales of the EORTC QLQ-PR25 using a confirmed analysis, and time to deterioration in EQ-VAS.41
Secondary endpoints in the ARAMIS trial came out in favour of darolutamide versus placebo; however, because of the hierarchical structure implemented, no conclusions can be made about the statistical significance of these endpoints until the final analysis, planned to occur after 240 deaths from any cause.22 Risk for pain progression (BPI-SF) was reduced by 35% versus placebo, with median time to pain progression of 40.3 months and 25.4 months, respectively (nominal p<0.001). Risks for cytotoxic chemotherapy and first symptomatic skeletal event were also reduced. Exploratory endpoints including time to PSA progression, time to first prostate cancer-related invasive procedure, and time to first subsequent antineoplastic therapy demonstrated improvements with darolutamide versus placebo (all nominal p<0.001). In terms of HRQoL, FACT-P scores, BPI-SF pain severity, and EORTC QLQ-PR25 urinary symptoms were improved with darolutamide versus placebo but did not reach clinically meaningful thresholds.22
The Influence of Next-Generation Imaging
Since the imaging protocols for the Phase III studies discussed above were designed, new techniques have been introduced that provide unprecedented accuracy for whole-body staging of prostate cancer. One technique using PET or CT targets the surface expression of prostate-specific membrane antigen (PSMA) ligand on prostate cancer cells. PSMA PET/CT has proven to be a valuable tool, not only for superior detection of nodal metastases even in patients with very low PSA levels (<2 ng/mL), but also for restaging patients in biochemical failure who have recurrent nodal disease.42,43
Whole-body MRI (wbMRI) has also been adopted for detection of biochemical recurrence, offering unrivalled soft-tissue contrast and the benefits of diffusion-weighted imaging. A prospective comparison of the two techniques concluded that PSMA PET/CT is superior to wbMRI for detection of lymph node metastases in men who have undergone radical prostatectomy. However, PSMA PET/CT is not without its flaws. Around 5% of prostate cancers do not express PSMA at detectable levels, and false-negative scans may arise due to masking of lesions adjacent to or within organs with a high level of background activity.44
Given the limitations of the imaging techniques used in the Phase III studies (i.e., SPARTAN, PROSPER, and ARAMIS), it is pertinent to question the degree to which patient staging may have shifted if PSMA PET/CT and wbMRI had been used instead. Taken together, improvements in detection of biochemical recurrence demonstrated in studies comparing choline PET/CT and wbMRI with bone scintigraphy45-52 and PSMA PET/CT with choline PET/CT53 suggest that many patients with nmCRPC on conventional imaging (ciM0) in the Phase III studies may in fact have been metastatic based on molecular imaging (miM1). This idea is supported by a recent analysis using PSMA-PET to detect distant metastatic disease in a patient population similar to that of the SPARTAN study.54 Restaging from ciM0 to miM1 was reported in 55% of patients, with lymph nodes being the most common metastatic site. Shared baseline characteristics suggest that ciM0/miM1 patients in both studies were at similar points in their disease’s natural history.9,54 This raises the question of how to manage patients with stage migration under more advanced imaging. Given that the indication for treatment was the same for ciM0 and miM1 patients within the context of SPARTAN and PROSPER,9,10 similar efficacy can be expected with apalutamide with ongoing ADT or enzalutamide in both groups.
Conclusion
Development of metastasis is a key turning point in the clinical course of prostate cancer and delaying time to metastatic progression is an important treatment goal in nmCRPC. As in other oncology settings where obtaining estimates of OS in Phase III trials is problematic because of logistical issues, the need for ICE is now recognised in nmCRPC. Phase III trials using MFS as a primary endpoint have demonstrated the effectiveness of apalutamide, enzalutamide, and darolutamide in delaying time to metastatic progression or death in men with high-risk nmCRPC. Based on the primary analyses, apalutamide and enzalutamide have been approved for the high-risk indication in Europe, and a marketing authorisation has been filed for darolutamide. These approvals are the first to be based on MFS, asserting its prognostic value in nmCRPC and underlining the physical and emotional benefits to patients of living metastasis-free. The observation that the HRQoL of men with nmCRPC (a disease state characterised by lack of symptoms) can be maintained or even improved by novel anti-androgens is encouraging. Perhaps of utmost importance, the finding that apalutamide with ongoing ADT is associated with significant improvement in time to second progression suggests that earlier treatment of nmCRPC may be more effective than waiting for metastatic progression before intervening.55
Although no single trial showed a significant increase in OS owing to too few survival events, two meta-analyses of SPARTAN and PROSPER were able to demonstrate a 24% reduction in the risk of death versus placebo. Additional follow-up from the Phase III trials (and potentially further meta-analyses of patient-level data) may help to clarify the possible OS advantage of early treatment in nmCRPC and provide further validation of MFS as a strong surrogate endpoint.








