Abstract
The epidemiology of scrotal cancer has changed over time away from occupational exposure to soot. The current incidence of scrotal malignancy is approximately 1 per 1 million males per year. This review summarises the current literature on the management of scrotal squamous cell carcinoma (SCC), including pathogenesis, available diagnostic tools, current treatment, and overall management strategies.
The rarity of SSC cases makes it difficult to recruit patients for studies of this disease. To date, very few studies have been performed, and those that have been completed were limited by a small sample size. This review analyses all available evidence, which varies from retrospective case series to prospective multicentre trials.
Psoralen ultraviolet light A treatment and human papillomavirus infection are significant risk factors for this cancer. Scrotal SCC had lower survival rates compared with other histological subtypes and the 5-year relative survival rate was 77%. Many studies also showed a positive margin, even after wide excision of the lesion. Excision of the primary lesion and a risk-stratified approach for staging and treatment of regional lymph nodes is the mainstay of current management strategies. For patients with clinically negative lymph nodes, sentinel lymph node biopsy and PET scans for patients with suspected pelvic node involvement has improved the diagnostic yield. The new neoadjuvant therapy (both chemotherapy and radiotherapy) has helped to downstage the disease for complete resection.
The prognosis of scrotal SCC is determined by margin-free excision, depth of infiltration, and its histologic grade. Future trials focussing on the conjunction of SCC with penile cancer, as well as the creation of a multinational network for ‘virtual’ online multidisciplinary meetings, will help to improve the overall survival for scrotal SCC patients.
INTRODUCTION
Chimney sweeps’ carcinoma was first described by Percivall Pott in 1775 who noted that the cancer was associated with occupational exposure to soot.1 The cancer primarily affected chimney sweeps who had been in contact with soot since early childhood. The median age at the onset of symptoms was 37.7 years, although boys as young as 8 years old were found to have the disease.2
It was proposed by W.G. Spencer in 1890 that sweat running down their bodies had caused soot to accumulate in the rugae of the inferior surfaces of the scrotum, with the resulting chronic irritation causing scrotal cancer.3 Back in 1840, a lengthy law was passed making it illegal for anyone under the age of 21 years to sweep chimneys and, in 1875, another law was passed that allowed only registered people to undertake the work, putting an end to the use of young chimney sweeps. These laws have decreased the incidence and changed the aetiology of scrotal cancer over time.3 The current incidence of scrotal malignancy is approximately 1 per 1 milllion males/year, the same as for penile squamous cell carcinoma (SCC). The rarity of cases of this disease and our understanding of the changing nature of scrotal cancer is the focus of this review, which will summarise the current literature in terms of current scrotal SCC pathogenesis, diagnostic evaluation tools, current treatments, and overall updated management.
Scrotal cancer has a number of histologic sub types: SCC (35%), extramammary Paget’s disease (22%), sarcoma (20%), basal cell carcinoma (17%), and melanoma and adnexal skin tumours (6%). SCC is the most common variety. The median (95% confidence interval [CI]) overall survival for localised low-risk scrotal cancers (basal cell carcinoma, extramammary Paget’s disease, sarcoma) and localised high-risk scrotal cancers (melanoma, SCC, adnexal skin tumours) was 166 (145–188) and 118 (101–135) months, respectively.4 Patients with regional and distant disease were reported to have poorer overall survival.5
The management and follow-up plans for scrotal SCC patients are not well documented in the literature. Surgical excision is the recommended treatment for localised disease, with confirmation of margin clearance. Wound closure can be performed with primary closure, skin grafts, flaps, or by secondary intention. Clear understanding of the management and survival outcomes of this disease can help the urologist to determine the appropriate course of treatment and patient care.
PATHOPHYSIOLOGY
Scrotal SCC was the first malignancy recorded to be linked directly to occupational exposure. Scrotal SCC has also been linked to exposure to tar, pitch, different types of lubricating and cutting oils, creosotes, gas production, and paraffin wax pressing.6-11 As there has been a huge improvement in both working environments and the relevant laws in place, occupational risk factors have not been associated with increased risk of scrotal SCC over the last two decades.12 A nationwide case control study found a significantly increased risk (odds ratio [OR]: 6.7; 95% CI: 1.0–45.6) of developing scrotal SCC with a cumulative lifetime duration of nude sunbathing of 26–150 hours. In addition, the results suggested that the use of sunbeds increases the risk of scrotal SCC (OR: 3.2; 95% CI: 1.0–10.4).13
Moreover, iatrogenic ultraviolet radiation for the treatment of skin diseases was also associated with a high risk of scrotal SCC. In a cohort of men with psoriasis, who had been treated with oral psoralen and ultraviolet A photochemotherapy (PUVA) and followed-up for 12.3 years, Stern et al.14 found a risk ratio of 95.7 (95% Cl: 43.8–181.8) for genital SCC in PUVA treated patients compared with the general population incidence rates. In patients exposed to high doses of PUVA, the incidence of invasive SCC was 16-times higher (95% CI: 9.4–26.4) than that of patients exposed to low doses. With a further 10 years of follow-up, Stern et al.6 found a 52.6-fold (95% CI: 19.3–114.6) increase in the incidence of genital SCC in this cohort compared with that expected for the general Caucasian population. Conversely, the link between PUVA and SCC was not confirmed in European prospective studies with relatively short follow-up periods;15 however, analysis of a large database, with an observation period of at least 14 years, showed a relative risk of 5.6–6.5 for SCC in European men.16,17 The male genitalia seem to be more susceptible to the carcinogenic effects of PUVA than non-genital areas. The carcinogenic effects of the PUVA therapy include DNA damage accumulation and immunosuppression.18 There was no increased risk of skin cancer reported in studies assessing the carcinogenic risk of narrow band ultraviolet B, which is used more commonly for treating psoriasis.19
Human papillomavirus (HPV) was previously linked to scrotal SCC. In a study of 14 patients at the Mayo Clinic, 6 (42%) had a history and histologic evidence of HPV infection.5 Matoso et al.11 evaluated a total of 29 cases of SCC of the scrotum in three North American institutions. These cases occurred between 1999 and 2013. Of 26 cases with available tissue, 7 (27%) tested positive for high-risk HPV serotypes using in situ hybridisation. Cases associated with HPV-infected disease displayed a predominantly basaloid or warty morphology and were characterised by p16 and Ki-67 immunostaining. Similar morphologic and immunohistochemical results in HPV-infected scrotal SCC patients suggested a similar pathogenic pathway to that proposed for penile SCC.20 Furthermore, scrotal SCC may be a manifestation of cutaneous carcinoma risk in immunodeficient patients. Matoso et al.11 also found that 5 out of 29 patients with SCC of the scrotum had immuno compromised conditions, such as with infection with HIV, after transplantation, and in leukaemia (Tables 1 and 2).
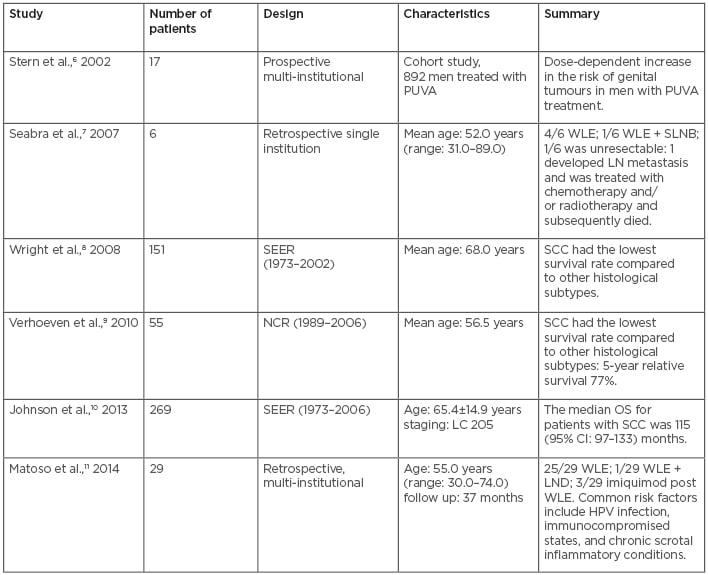
Table 1: Published case series with pathophysiology and management of scrotal squamous cell carcinoma.
DD: distant disease; LC: local disease; LN: lymph node; LND: lymphadenopathy; NCR: Netherlands Cancer Registry; OS: overall survival; PUVA: psoralens and ultraviolet A radiation; RL: regional lymph node; SCC: squamous cell carcinoma; SEER: surveillance, epidemiology and end results; SLNB: sentinel lymph node biopsy; WLE: wide local excision.
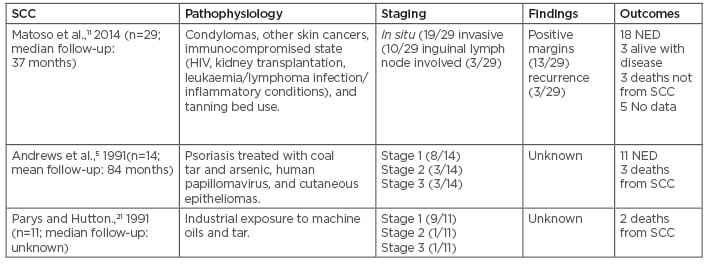
Table 2: Squamous cell carcinoma treatment outcome with pathophysiology.
NED: No evidence of disease; SCC: squamous cell carcinoma.
Recognised risk factors for SCC include:
- Occupations such as chimney sweeps and tar and paraffin workers. Also, occupations with exposure to mineral and cutting oils, printing, and metal working, such as car and aeroplane manufacturers, car mechanics, commercial printers, aluminum workers, shale oil workers, pitch workers, engineers, steel production workers, and cavalry personnel.
- Exposure to carcinogenic metals (e.g., arsenic, nickel, and chromium).
- Chronic mechanical irritation.
- Chronic inflammatory states (e.g., chronic lymphoedema and surgical scars).
- Lifestyle factors (e.g., poor personal hygiene and smoking).
- Viruses (e.g., HPV).
- Exposure to ionising radiation.
- Exposure to iatrogenics (e.g., coal, tar, PUVA, radiotherapy, nitrogen mustard, and Fowler’s solution).
- Immunosuppression (e.g., acquired and inherited immunodeficiency, and post-transplant immunosuppression).
DISEASE STAGING
The most commonly used staging system is the Lowe modification of the system proposed by Ray and Whitmore.22 It is based on the extent of local disease and the level of metastasis (Table 3). Diagnostic evaluation of scrotal cancer also depends on various pathologic subtypes and proper disease staging. Inguinal lymph nodes are common sites for metastasis in cases of invasive disease.
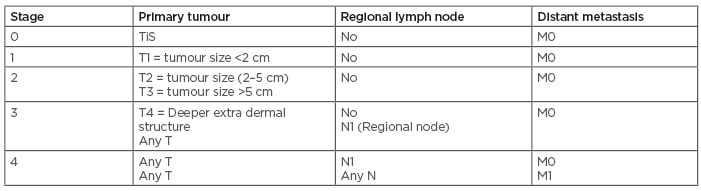
Table 3: Staging scrotal cancer.
M: metastasisation; dN: number of affected nearby lymph nodes; N: node; T: tumour; TiS: carcinoma in situ.
CLINICAL EXAMINATIONS OR INVESTIGATIONS
Excisional biopsy is required for the lesions to determine the histology of the scrotal cancer. Evaluation of non-localised disease and metastases can be performed through careful physical examination and cross-sectional imaging modalities, such as CT scanning or MRI; however, MRI is better for soft tissue lesion assessment. PET scanning should be considered if there is suspected pelvic lymph node involvement or beyond.23-27
Advanced disease may invade the testes or penis. This diagnosis is confirmed by histologic evaluation, and several areas should be sampled to determine the boundary of extension and depth of invasion. The scrotum has the same lymphatic drainage pattern as the penis. Tumours usually spread stepwise from the inguinal lymph nodes to the pelvic lymph nodes.
Interestingly, the scrotal lymphatics do not appear to cross the median raphe and drain into the ipsilateral superficial inguinal lymph nodes. Therefore, tumours without involvement of the median raphe rarely metastasise to the opposite inguinal site.23 Because of similarities in location and histology, the clinical workup for scrotal SCC is quite similar to that of penile cancer. Routine imaging examinations include pelvic and/or abdominal CT scans and chest radiographs. Other tests, such as chest CT or PET-CT and bone scans, may be used when indicated.23-27
LYMPH NODE MANAGEMENT
Physical examination and cross-sectional imaging techniques are inaccurate for determining lymph node metastasis in cases of invasive scrotal SCC.24 An inflammatory reaction may cause enlargement of the inguinal lymph nodes and fine needle aspiration cytology is the easiest way to confirm metastasis; however, the examination is only helpful if the results are positive. If the fine-needle aspiration cytology is negative and a lymph node is still palpable after antibiotic treatment, an excision biopsy is advised.25 In cases of clinically negative inguinal nodal basin, approximately 23% of patients will harbour occult metastases.26 However, ultrasound with fine-needle aspiration cytology reportedly failed to identify 35% of inguinal sampling with metastatic lymph nodes.26 On the contrary, the addition of dynamic sentinel lymph node biopsy increased the detection rate to 95% and can serve as an alternative to prophylactic lymph node dissection in dedicated centres.26 For pelvic lymph node metastases, fluorodeoxyglucose PET-CT scan reportedly showed a sensitivity of 91%, a specificity of 100%, and a diagnostic accuracy of 96% in a pilot study of 28 pelvic basins.27,28
MANAGEMENT AND OUTCOME
The primary treatment modality for scrotal carcinoma is surgery. Treatment for all varieties of histology requires surgical removal of the malignancy. Adjuvant treatments, including radiation therapy and chemotherapy, can be considered. Dai et al.29 reported a case series of 10 patients with scrotal carcinoma in which all patients were treated with wide surgical excision only. After an average follow-up of 47 months, 8 patients were in good health without any recurrence. One patient developed left inguinal lymph node metastasis at 21 months that was successfully treated with bilateral inguinal lymphadenectomy, and the final patient developed bilateral pulmonary metastasis at 48 months and was palliatively treated with chemotherapy.29 Neoadjuvant therapy (both chemotherapy and radiotherapy) has also been recommended to downstage (reduction of the tumour size and lymph node status, thus improving the stage of the disease) a very large lesion to achieve complete resection.30,31 Adjuvant RT in combination with chemotherapy (methotrexate, bleomycin, and cisplatin) for four cycles is also recommended to achieve better disease-free survival.32
The prognosis in scrotal SCC depends on various factors, such as the age of the patient; size, grade, and stage of the tumour; and extent of surgery. It has been reported that the status of surgical margin is an important predictor for local and/or regional tumour control. It has also been reported that the surgical margin is still positive despite wide excision with a 2 cm margin.33 Frozensection could have been a potential solution to this situation; however, no studies have mentioned this important diagnostic tool. Treatment outcomes of scrotal SCC in published case series are shown in Table 2.
In summary, for a patient with scrotal carcinoma and inguinal lymph node metastasis, surgical treatment followed by adjuvant chemo-radiotherapy may achieve palliative tumour control and symptom relief; however, close follow-up is warranted to evaluate long-term treatment management. The author has published a case report of a patient in whom scrotal cancer had clear blood supply from the spermatic cord. The patient was followed-up for 5 years to check for recurrence.34
FOLLOW-UP PLANNING
Every patient should be followed-up with history and physical examination (including skin) every 3 months for 2 years, then every 6 months for 3 years, then annually for life.35 Patient education regarding sun protection and self-examination of the skin for regional disease recurrence needs to be mentioned during consultations.
Any recurrence should be treated by local excision. For new regional disease, regional lymph node dissection should be considered.35 Regional recurrence or distant metastases should be reviewed and discussed in a multidisciplinary team setting for consideration of combined chemo-radiotherapy. Depending on the literature, the 5-year overall survival rates for low-risk risk scrotal cancers (sarcoma, extramammary Paget’s disease, and basal cell carcinoma) and high-risk scrotal cancers (melanoma, SCC, and adnexal tumours) are about 75% and 55%, respectively.35
EMERGING TREATMENT
The era of targeted molecular and immuno therapies holds promise for the management of advanced SCC.36 Cetuximab, an anti-epidermal growth factor receptor (EGFR) monoclonal antibody, is now an approved agent for treatment of head and neck SCC.37 Emerging therapies for head and neck SCC include EGFR tyrosine kinase inhibitors, vascular endothelial growth factor receptor inhibitors, insulin-like growth factor receptor inhibitors, and inhibitors of the PI3K/AKT/mTOR pathway, which may have a role in the treatment of patients with scrotal SCC in the future.38 However, current data on the efficacy of such therapies for patients with scrotal SCC is lacking. Recently, Lavens et al.39 showed increased EGFR expression in penile SCC. Carthon et al.40 evaluated EGFR targeted therapy in patients with advanced penile or scrotal cancer in a retrospective case series of 24 patients. Only 1 of 24 patients had scrotal SCC. This patient developed metastases to the right groin with disease progression despite paclitaxel, ifosfamide, and cisplatin chemotherapy. The addition of EGFR-targeted therapy led to a reduction in tumour burden and thereby allowed resection of metastatic deposits. This was the only patient reported to be disease-free 38 months post EGFR therapy.40 Further correlative-biology studies are needed to establish EGFR status in scrotal SCC tissue and response to EGFR targeted therapies in a prospective fashion. Due to the low incidence of scrotal SCC, a multicentre collaboration would be needed. Further genomic and molecular characterisation of scrotal SCC will be important in identifying key pathways and developing therapeutic targets in the future.
CONCLUSION
Although historically considered an occupational disease, the epidemiology of scrotal SCC has changed in recent years. Nowadays, iatrogenic conditions, such as PUVA and immuno suppression, and HPV infection play a significant role in the risk of developing this cancer. Surgery is the mainstay of the treatment algorithm for scrotal SCC. Excision of the primary lesion and a risk-stratified approach for the staging and treatment of regional lymph nodes is advisable. For patients with high-risk disease and negative clinical lymph nodes, sentinel lymph node biopsy can mitigate the morbidities of full groin dissection and possibly identify those with early occult disease.24-27 For patients with suspected pelvic node involvement, PET scanning is useful. For locally advanced and metastatic disease, palliative chemotherapy is advocated. Given the rarity of this condition, multicentre trials in conjunction with trials for the management of penile SCC are likely to provide further knowledge in this field for future urologists.








