Abstract
Introduction: The use of angiotensin-converting enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARB) has been associated with improved bladder cancer outcomes. The objective of this study was to perform a systematic review of the literature and investigate the effects of these medications on survival from our own retrospective database.
Methods: A systematic literature search of PubMed and the Cochrane database was conducted and 34 relevant articles identified. No randomised control trials were identified. After exclusion, five observational studies were included in our analysis. Since there was a paucity of data, we then performed a retrospective cohort study using clinical data from our electronic medical record. All patients who underwent radical cystectomy, with or without adjuvant chemotherapy, at a single tertiary care centre in Ontario, Canada between 2001 and 2016 were identified.
Results: Our literature review found that ACEI or ARB use in upper urinary tract and lower urinary tract non-muscle invasive bladder cancer was associated with increased 5-year recurrence-free, cancer-specific, and overall survival. Our own analysis identified 464 patients who underwent radical cystectomy for muscle-invasive bladder cancer during the study period. Ninety-nine individuals received ACEI or ARB treatment during this time. Cox-proportion hazards modelling suggested that the use of ACEI or ARB was not significantly associated with a survival benefit.
Conclusions: We are unable to support or oppose the use of ACEI or ARB as adjuvant treatment in bladder cancer due to the heterogeneity and quality of published data. Our own study data do not support the use of these medications as adjuvant therapy for muscle-invasive bladder cancer. A randomised control trial in this area of research is required.
INTRODUCTION
Bladder cancer is the ninth most common cause of cancer worldwide and was associated with 165,000 deaths in 2012.1 The incidence of bladder cancer is much higher than its associated mortality rates, but it is among the most expensive cancers to treat and monitor, with a significant proportion of the costs attributable to high rates of recurrence and progression.2 Although high-quality evidence supports intravesical Bacillus Calmette–Guérin (BCG) therapy and neoadjuvant chemotherapy to prevent disease recurrence in non-muscle invasive bladder cancer (NMIBC)3 and muscle-invasive bladder cancer (MIBC),4 recurrence rates remain high. There has been considerable interest in the identification of adjuvant treatments to improve disease outcomes.
Angiogenesis, induced by angiotensin II (AngII) and other factors, is necessary for tumour growth.5 Angiotensin II Type 1 receptor (AT1R) is associated with various malignancies, including melanoma, pancreatic cancer, breast cancer, and bladder cancer.6,7 Its role in angiogenesis, growth, and metastasis of tumour cells is well-established.8,9 Activation of this receptor via the AngII–AT1R signalling pathway leads to vascular endothelial growth factor (VEGF) expression, stimulating tumour angiogenesis. Furthermore, the AngII–AT1R signalling pathway promotes macrophage mobilisation and infiltration into the tumour bed, mainly secondary to monocyte chemoattractant protein-1.7 This chemokine acts as a prominent regulator of tumour growth, aggression, and migration, and ultimately promotes tumour cell survival.8 The AngII–AT1R signalling pathway has been shown to cause increased expression of VEGF. Since the AngII–AT1R signalling pathway upregulates VEGF, the suppression of Ras may impede or even prevent angiogenesis, which is necessary for sustained tumour growth.6,7,10 Therefore, it is speculated that the use of angiotensin-converting enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARB) may influence the outcome of malignant tumours, including bladder cancer.8,11 The objective of this study was to perform a systematic review of the literature and investigate the effects of ACEI and ARB use on survival from our own retrospective database.
METHODS
Phase 1
A systematic review of the literature was performed according to the methods developed by the GRADE Working Group.12-14 A focussed clinical question was formulated that directed comprehensive literature searches for high-quality research articles. All study types were considered due to the paucity of data. Disease-specific and overall survival (OS) were considered critical outcomes of interest; disease recurrence was considered a secondary outcome of interest.
A systematic literature search was conducted on PubMed (1966–2017) using the MeSH search terms: “Urinary Bladder Neoplasms”[MeSH] AND “Angiotensin Receptor Antagonists”[MeSH]. The search was limited to clinical trials with a human population in the English language. The Cochrane database was also searched for relevant high-quality systematic reviews on this topic. A snowballing technique was used from identified articles and recent published systematic reviews within this area to ensure a comprehensive search to identify additional articles for inclusion within our systematic review.
Study information was abstracted by a single reviewer and entered into RevMan. The quality of evidence informing each clinical question was rated on an outcome-specific basis as high, moderate, low, or very low according to GRADE.12 Dimensions of evidence quality considered were risk of study bias, inconsistency, indirectness, imprecision, and publication bias. These steps were performed independently by two reviewers; disagreements were resolved by discussion. Abstracted data were subsequently transferred into GRADEPRO and used to generate evidence profiles in the standard GRADE format.
Phase 2
It was established that there was a lack of high-quality data regarding outcomes associated with ACEI and ARB use in bladder cancer; thus, this review was augmented with an analysis from the authors’ own dataset.
Study Design and Setting
A retrospective cohort study was conducted using clinical data from an electronic medical record. All residents of Ontario (population: 13 million) have access to a government-administered single-payer healthcare system.
Population
All patients who underwent radical cystectomy, with or without adjuvant chemotherapy, were identified at a single tertiary care centre in Ontario, Canada, between 1st August 2001 and 30th June 2016. There were no exclusion criteria.
Outcomes and Exposures
The primary outcome was survival, defined using death as documented in the authors’ electronic medical records from the time of cystectomy. The main exposure of interest was use of ACEI or ARB identified by chart review. There were several other exposures also included.
Demographic characteristics
Sex, age, American Society of Anesthesiologists (ASA) score, medical comorbidities (cardiovascular, metabolic, musculoskeletal, gastrointestinal, pulmonary, genitourinary, neurological, and oncological), previous surgery, previous pelvic radiation, presence of radiation cystitis, lifetime smoker status, current smoker status, medication history (metformin, pioglitazone, rosiglitazone, statin, renin-angiotensin inhibitor), and vegetarian status.
Perioperative characteristics
Number of transurethral resections of bladder tumours (TURBT), number of bladder lesions on cystoscopy, localisation of bladder lesions, histological diagnosis from TURBT, date of referral from primary caregiver, symptoms at initial consultation with a urologist, date of cystectomy, perioperative blood loss, hospital stay length, operative time, urinary diversion type, neoadjuvant treatment status, and adjuvant treatment status.
Postoperative characteristics
Cystectomy histopathology, tumour grade, pathologic T-stage, presence of non-invasive flat carcinoma, number of positive nodes, presence of prostate cancer, pathologic T-stage of prostate cancer, presence of prostatic intraepithelial prostate cancer, presence of atypical small acinar proliferation within the prostate, proportion of the prostate involved by primary tumour, Gleason score, presence of positive margins, number of lymph nodes, presence and grade of tumour in ureter on frozen section, and tumour extension into the vagina and/or urethra.
Follow-up characteristics
Postoperative pelvic radiation, recurrence of cancer, date of recurrence, pain at recurrence, site of recurrence (lymph node, pelvis, bowel, liver, lung, and bone), death, cause of death, and date of last follow-up.
Statistical Analyses
Baseline variables are reported as medians (interquartile range [IQR]) or counts (percentages) and were compared using student’s T-test for continuous variables, and Chi-square test for categorical variables. A two-sided p<0.05 was considered significant. Differences in survival rates were assessed using Kaplan–Meier survival estimates with Cox-proportion hazards modelling.
RESULTS
Phase 1
The clinical question constructed was: ‘Do angiotensin converting enzyme inhibitors or angiotensin II receptor blockers affect bladder cancer outcomes?’
Literature Search
A total of 41 articles were identified for review. Of these 41 articles, after reviewing the title and abstracts, 35 articles were removed for non-relevance. The remaining 6 articles were comprehensively reviewed, and 1 was excluded for for non-relevance, leaving 5 articles for inclusion in this study.
Evidence
No randomised control trials were identified. A total of five observational studies were included in the analysis; these studies examined the effect of ACEI or ARB use on outcomes related to several components of the urinary system.
Two studies examined outcomes associated to lower urinary tract NMIBC post-TURBT. Blute et al.6 performed a retrospective cohort study of 340 patients with NMIBC post-TURBT, identified using an institutional bladder cancer database. It was found that ACEI or ARB use was associated with reduced tumour recurrence (relapse-free survival [RFS]) (hazard ratio [HR]: 0.61; 95% confidence interval [CI]: 0.45–0.84; p=0.005) but not improved progression-free survival (HR: 0.6; 95% CI: 0.19–1.97; p=0.400) on multivariate analysis. A Kaplan–Meier analysis showed that 5-year RFS was 45.6% in individuals who received ACEI or ARB compared to 28.1% in those who did not (p=0.01). Yuge et al.9 performed a retrospective cohort study of 330 patients with NMIBC post-TURBT, identified on chart review. They found that lack of ACEI or ARB use was associated with worse RFS (HR: 2.26; 95% CI: 1.22–4.19; p=0.010) but not associated with risk of stage progression on multivariate analysis. Kaplan–Meier analysis showed that 5-year RFS rate was 78.4% in individuals who received ACEI or ARB compared to 53.3% in those who did not (p=0.017).
One identified study analysed outcomes associated with lower urinary tract MIBC radical cystectomy. Yoshida et al.15 performed a retrospective cohort study of 216 patients post-radical cystectomy from chart review at three institutions. The study found that ACEI or ARB use was associated with improved cancer-specific (CSS) (HR: 0.47; 95% CI: 0.23–0.95; p=0.036) and OS (HR: 0.36; 95% CI: 0.18–0.73; p=0.02) on multivariate analysis. Kaplan–Meier analysis showed that the 10-year OS rate was 71.3% in individuals who received ACEI or ARB compared to 47.7% in those who did not (p=0.0097).
Two studies were identified examining outcomes associated with upper tract urothelial carcinoma post-nephroureterectomy (nephroU).16,17 Yoshida et al.16 performed a retrospective cohort study of 312 patients post-nephroU identified on chart review at four institutions. They found that ACEI or ARB use was associated with improved RFS (HR: 0.48; 95% CI: 0.27–0.87; p=0.015), CSS (HR: 0.31; 95% CI: 0.15–0.65; p=0.002), and OS (HR: 0.45; 95% CI: 0.26–0.78; p=0.004) on multivariate analysis. Kaplan–Meier analysis demonstrated 5-year OS was 68.7% in individuals who received ACEI or ARB compared to 61.8% in those who did not (p=0.047). Tanaka et al.17 performed a retrospective cohort study of 279 patients post-nephroU on chart review at two institutions. It was found that a lack of ACEI or ARB use was associated with worsened metastases-free survival (HR: 3.14; 95% CI: 1.14–8.67; p=0.027) but not associated with CSS on multivariate analysis. Kaplan–Meier analysis showed that 5-year OS was 85.8% in individuals who received ACEI or ARB compared to 73.7% in those who did not (p=0.047).
These studies were then evaluated for quality using GRADE criteria. The critical outcomes for this question were defined as related to OS, disease recurrence, and disease progression or metastasis-free survival. No data on complications were presented in any of the studies (Table 1).
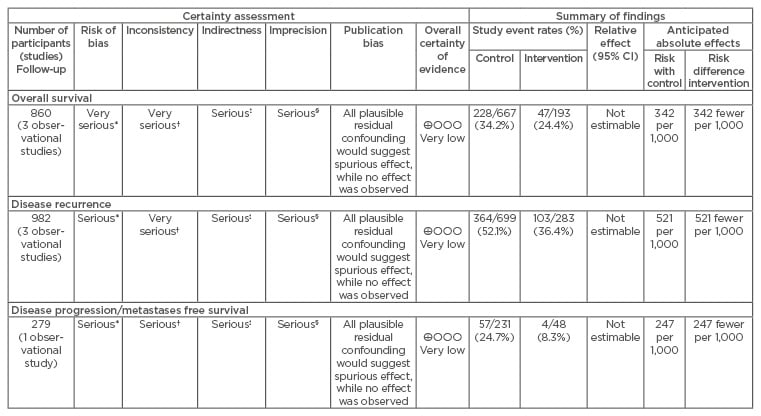
Table 1: GRADE evidence profile for the question: Do angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers affect bladder cancer outcomes?
*Retrospective cohort study; †different disease sites; ‡different interventions between groups; §small overall number of participants.
CI: confidence interval.
Clinical Implications
Unfortunately, a recommendation cannot be made that either supports or opposes the use of ACEI or ARB as adjuvant therapy for bladder cancer, because of the low quality and heterogeneity of the published data. The results presented within this literature review are encouraging that these medications could offer a significant survival benefit with a minimal and well-established toxicity profile. Randomised control trial data are needed within this area of research.
Phase 2
A total of 464 patients were identified who underwent radical cystectomy during the study period, of whom 354 (77.5%) were male and were of median age of 71 years (IQR: 63–76) and median ASA score of 3 (IQR: 3–3). Patients had a variety of comorbidities, including cardiovascular disease (66.70%; most commonly hypertension, which appeared in 152 [44.00%] patients), metabolic disease (65.00%; most commonly hypercholesterolaemia, which appeared in 72 [21.50%] patients), genitourinary disease (13.20%; most commonly benign prostatic hyperplasia, which appeared in 34 [12.45%] patients), and pulmonary disease (12.90%; most commonly chronic obstructive pulmonary disease, which appeared in 28 [10.00%] patients). Approximately half (n=182 [42.30%]) of patients had previously smoked and 21.85% (n=94) continued to smoke at the time of surgery. Patients commonly presented with haematuria (n=115 [44.7%]) or multiple symptoms, including frequency, urgency, or urinary retention (n=127 [49.4%]). Preoperatively, 22.6% of patients (n=99) received neo-adjuvant chemotherapy. Statistical analysis revealed no difference between the total cohort, patients with disease recurrence, or patients who died during the study period for these characteristics (Table 2).
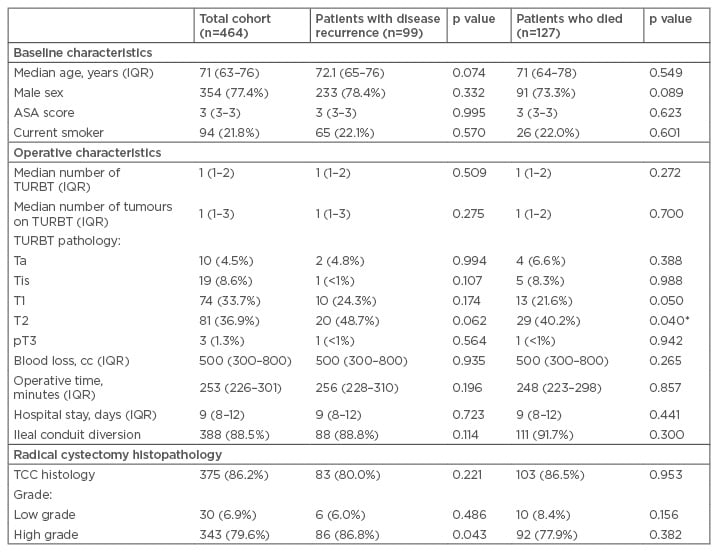
Table 2: Cohort baseline characteristics.
*Statistically significant.
ASA: American Society of Anesthesiologists; IQR: interquartile range; TCC: transitional cell carcinoma; TURBT: transurethral resection of bladder tumours.
The majority of patients had undergone a single TURBT (median: 1; IQR: 1–2) with a median of 1 bladder tumour (IQR: 1–3) most commonly located at the trigone (n=19 [11.0%]) and most commonly Stage T2 (n=81 [36.9%]). During radical cystectomy, median blood loss was 500 cc (IQR: 300–800) with a median operative time of 253 minutes (IQR: 226–301). The most commonly performed urinary diversion was an ileal conduit (n=388 [88.5%]). Average hospital stay was 9 days (IQR: 8–12). Statistical analysis revealed an increased risk of having T1 or T2 tumour on TURBT among individuals who died during the study period as compared to the total cohort (Table 2).
Histopathology from RC most commonly showed transitional cell carcinoma (n=375 [86.2%]) of high grade (Grade 3; n=343 [79.7%]) and 122 individuals with positive nodes (28.9%). Thirty-five patients had positive tumour margins (8.2%) and 41.8% were found to have prostate cancer. Postoperatively, 18.1% of patients received chemotherapy. Statistical analysis revealed an increased risk of having a higher stage and grade tumour from RC pathology among individuals with disease recurrence, as compared to the total cohort (Table 2).
We identified 99 individuals who had received ACEI or ARB during the study period. Kaplan–Meier survival curves were generated comparing survival of individuals who had received ACEI or ARB versus those who had not. Cox-proportion hazards modelling indicated that ACEI or ARB use was not significantly associated with a survival benefit (HR: 1.21; 95% CI: 0.76–1.92; p=0.419) (Figure 1).
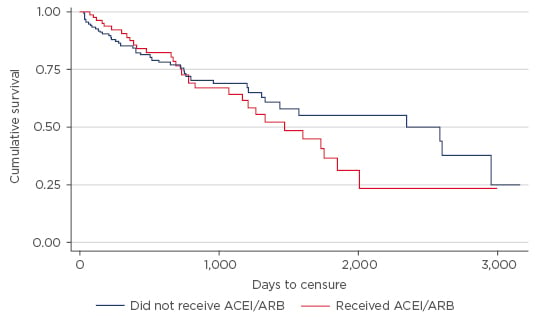
Figure 1: Kaplan–Meier curves from date of surgery to time of death or loss to follow-up by angiotensin-converting enzyme inhibitors or angiotensin II receptor blocker use.
ACEI: angiotensin-converting enzyme inhibitors; ARB: angiotensin II receptor blockers.
DISCUSSION
The literature and the authors’ own analyses are unable to support the use of ACEI or ARB to delay recurrence, prevent progression, or improve survival for individuals with bladder cancer. The systematic review of the literature identified that there is a paucity of high-quality literature to guide decision making within this area, because the published studies are of low quality, based on diverse clinical populations, and use a variety of outcomes to assess this clinical question.
There are several important limitations to this study. The authors’ own analysis is retrospective in nature, has a relatively short outcome period, and did not collect comprehensive data on the duration, quantity, or type of ACEI or ARB used. The literature review was confined to PubMed and the Cochrane database and was limited to English language papers only. These limitations highlight the need for a high-quality randomised control trial analysing this issue. Given the relatively modest cost associated with ACEI and ARB, this trial would not be overly expensive or require extensive regulatory review. The ideal dose and scheduling of medication would likely reflect current patterns for use in the treatment of hypertension, as per previous observational studies. These medications have well- established side-effect profiles and so should be relatively easy to identify.
Several meta-analyses have been published within this area but are, unfortunately, of very low quality and have provided no clarity for our clinical question. Several meta-analyses have examined whether the use of ACEI or ARB is associated with improved oncologic outcomes across a variety of cancers sites,18-20 but these studies have grouped the heterogenous articles we have identified and meta-analysed the resulting data; this is an inadequate methodological approach to the data within this area when aiming to guide clinical practice and must be viewed purely as hypothesis-generating. There is a clear need for high-quality randomised control trials within this area, given the encouraging results from observational studies that have been performed to date.