Abstract
A 44-year-old otherwise healthy male who had undergone left high inguinal orchidectomy, with histopathology suggestive of classical seminoma, was referred to the authors’ oncology centre for evaluation of persistently deranged renal function tests and initiation of chemotherapy. They had a large retroperitoneal mass encasing their left kidney and their creatinine was 4.2 mg/dL. Even 1 week after double J stenting, they had persistently elevated creatinine of 3.1 mg/dL, which was precluding their curative chemotherapy with bleomycin/cisplatin/etoposide regimen. In a desperate situation, to prevent further progression of disease, pre-phase chemotherapy with carboplatin and etoposide was considered. In anticipation of tumour lysis syndrome, considering the large mass and compromised renal function, a tumour lysis profile was requested, which revealed elevated serum calcium levels (15.4 mg/dL, which goes against the tumour lysis syndrome). Considering the large retroperitoneal lymph nodal mass, suppressed parathyroid hormone levels (4.1 pg/mL) and vitamin D3 being within normal range, a paraneoplastic cause of hypercalcaemia was considered. Correction of hypercalcaemia with medical measures as well as treatment of seminoma was instituted, which led to normalisation of renal function tests within the next 10 days. Here, the authors report a rare case of testicular seminoma with persistently deranged renal function, likely due to paraneoplastic hypercalcaemia, which improved after successful chemotherapy along with anti-hypercalcaemic measures, including aggressive hydration, diuretics, calcitonin, dexamethasone, and denosumab. This report shows that it is important to treat the cause along with medical management in this oncologic metabolic emergency. It also highlights the value of pre-phase chemotherapy with carboplatin and etoposide in the setting of acute renal impairment.
Key Points
1. Paraneoplastic hypercalcaemia leading to impaired renal function can rarely occur with seminoma presenting as a large retroperitoneal mass.
2. Although obstructive uropathy is a common and logical consequence of a large retroperitoneal mass accompanied by renal impairment, medical causes must be considered if successful stenting of ureters does not improve renal function.
3. Treatment of the hypercalcaemia and the seminoma with non-nephrotoxic chemotherapy such as carboplatin and etoposide leads to rapid resolution of both the renal impairment and the tumour.
BACKGROUND
Testicular seminomas are the most common type of testicular germ cell tumours.1 It typically affects young males between the ages of 30–55 years. Because of its high sensitivity to chemotherapy and radiotherapy, these tumours have high cure rates.2 Neoplasm is the most common cause of hypercalcaemia in hospitalised patients. Paraneoplastic hypercalcaemia affects about 20% of all patients with cancer.3 However, its incidence is very rare in testicular seminomas, with only few cases reported in the last five decades.4-9 Here, the authors report a case of seminoma complicated by paraneoplastic hypercalcaemia. This case report signifies the importance of treating the principal cause of hypercalcaemia along with the value of pre-phase chemotherapy with carboplatin and etoposide in germ cell tumours.
CASE PRESENTATION
A 44-year-old male was referred to the authors’ oncology setup for evaluation of deranged renal function tests (RFT) and for initiation of chemotherapy. One month prior to admission, they presented to their local hospital with history of abdominal pain, left testicular swelling, loss of appetite, and significant weight loss of 3 months duration. Ultrasonography of the abdomen and pelvis showed a large mass in the region of the left kidney, which was not seen as a separate structure. Sonography of the inguinoscrotal region showed enlarged (7.7×4.2×5.0 cm), lobulated left testis with altered echotexture suggestive of a testicular tumour. Their creatinine at this point of time was 3.39 mg/dL. In view of raised creatinine, a plain CT study of the abdomen and pelvis was done, which showed enlarged left kidney and a large retroperitoneal mass lesion measuring 15x14x12 cm, suggestive of lymph nodal metastasis. They previously underwent left high inguinal orchidectomy at a different hospital, which on histopathology was suggestive of classical seminoma.
Their chest X-ray was normal, their β-human chorionic gonadotropin was moderately raised (898.7 IU/L), and their α-fetoprotein was normal. Therefore, they were categorised as good risk seminoma according to the International Germ Cell Consensus Classification. The standard of care in good risk seminoma is chemotherapy with three cycles of bleomycin/cisplatin/etoposide (BEP) regimen. However, 3 weeks post-high inguinal orchidectomy, their serum creatinine remained elevated at 4.2 mg/dL, their blood urea nitrogen was 98.4 mg/dL, and this was deterring their further treatment with chemotherapy. The MRI study of abdomen and pelvis showed multiple enlarged discrete as well as conglomerate lymph nodal mass lesions involving retroperitoneum and left renal pelvis/renal fossa, with extensions across midline to right side threatening to encase the right ureter (Figure 1). Therefore, they underwent a cystoscopy and right double J (DJ)-stenting as a preventive measure.
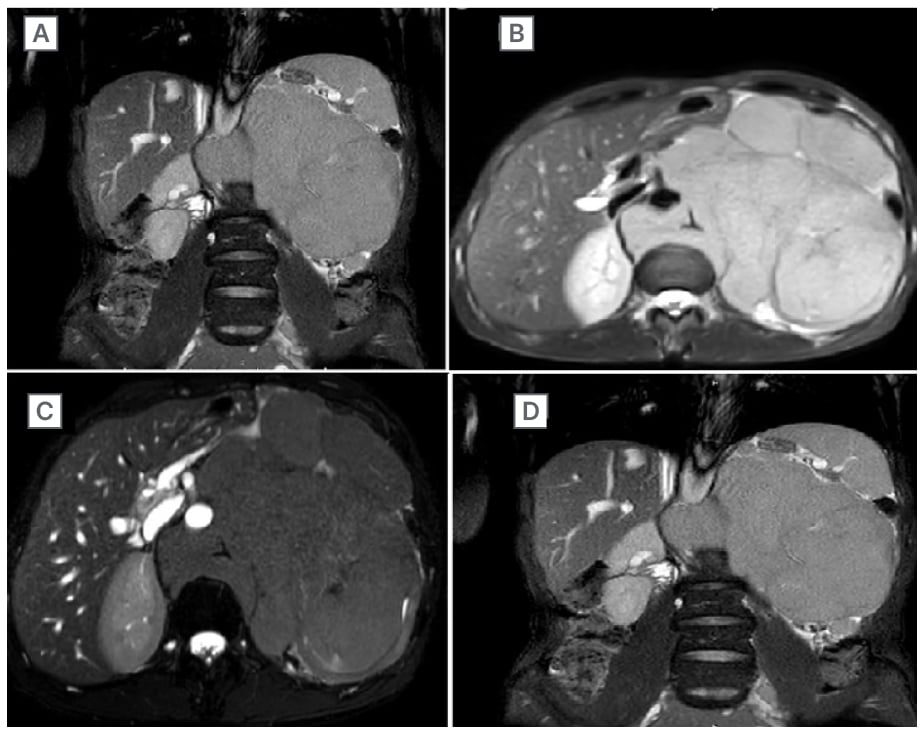
Figure 1: Image showing the retroperitoneal mass with extents.
A large retroperitoneal metastatic mass occupying and engulfing the kidney (A) and also crossing over to the right side (B). The mass has also engulfed and displaced the aorta with angle of contact >180° (C). The contrast-enhanced images in (C) and (D) show function loss of the left kidney.
With aggressive hydration, the serum creatinine improved to 2.9 mg/dL and blood urea nitrogen to 86.4 mg/dL. One week post-DJ stenting, they presented with complaints of back pain and loss of appetite. At this time, their serum creatinine was persistently elevated at 3.05 mg/dL, blood urea nitrogen at 106.10 mg/dL, lactate dehydrogenase at 593.00 U/L, and phosphorous at 5.70 mg/dL. They were referred to medical oncology for management of retroperitoneal mass with elevated lactate dehydrogenase and β-human chorionic gonadotropin in the context of a deranged RFT. The standard BEP regimen could not be initiated in the authors’ patient due to impaired renal function.
In anticipation of tumour lysis syndrome due to compromised renal function, a tumour lysis profile was done 7 weeks from diagnosis, which revealed an elevated calcium level at 15.4 mg/dL (corrected calcium level; calcium: 15mg/dL; albumin: 3.4mg/dL). Considering the clinical context, a paraneoplastic cause of elevated calcium was suspected, which was corroborated by parathyroid hormone (PTH) levels that were suppressed at 4.1 pg/mL (normal: 10.0-55.0 pg/mL) and vitamin D3 levels that were within normal range. A PTH-related peptide (PTHrP) assay was not done, as this investigation was not available at the authors’ hospital. The patient was initiated on antihypercalcaemic measures (aggressive hydration, calcitonin, furosemide, and denosumab), as well as pre-phase chemotherapy with carboplatin (a one-time 150 mg flat dose) and etoposide (100 mg flat dose for 3 days; etoposide requires 25% dose reduction if creatinine clearance is 15–50 mL/min; therefore, its adjusted renal dose is approximately 100 mg).
Their creatinine levels began to decline mirroring the fall in serum calcium (Figure 2). Their creatinine clearance (estimated by the Cockcroft-Gault method) improved from 12.95 mL/min to 56.60 mL/min. Corresponding to the decrease in corrected calcium and creatinine levels, an ultrasonography of abdomen and pelvis 6 days after the initiation of chemotherapy showed regression of mass from 17x13x12 cm to 17x7x11 cm. At discharge, their serum creatinine had come down to 1.04 mg/dL, urea to 44.3 mg/dL, phosphorous to 1.8 mg/dL and calcium to 8.5 mg/dL. They were subsequently planned for the standard three cycles of BEP as recommended for good risk seminoma. The DJ stent removal was done 3 days after their renal parameters stabilised. Their appetite improved as well, and their weight increased from 40 to 45 kg. A timeline chart representing the major events in this case is represented in Figure 2.
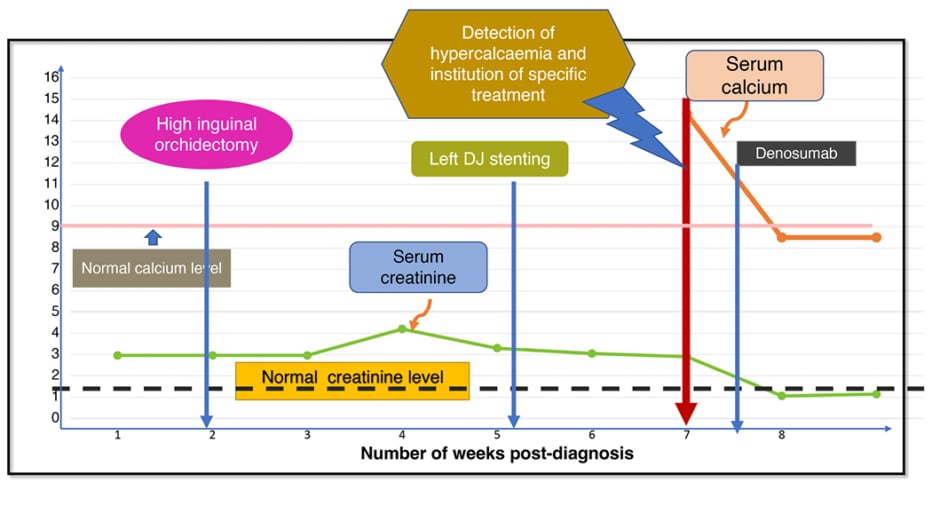
Figure 2: Timeline of events from diagnosis.
DJ: double J.
DISCUSSION
This case report is to highlight a rare metabolic cause of renal impairment due to hypercalcaemia in a patient with a testicular tumour and a large retroperitoneal mass. In the clinical situation of large retroperitoneal mass and deranged RFTs, the usual suspicion is of obstructive uropathy. Therefore, a right DJ stent was prophylactically placed to preserve right kidney function and prevent further renal impairment. However, these measures did not improve the renal functions and the raised creatinine levels remained an unsolved problem in this patient. They were incidentally detected to have hypercalcaemia on a tumour lysis profile, which is a feature against the occurrence of tumour lysis syndrome. In individuals with paraneoplastic hypercalcaemia, a low-to-normal PTH level, typically high PTHrP levels, along with elevated calcium levels, are the common laboratory findings.10 Although PTHrP levels were not determined in the authors’ patient, low PTH with normal vitamin D and elevated calcium levels indicated hypercalcaemia of malignancy, possibly due to secretion of PTHrP by tumour cells.
Since the likely cause of hypercalcaemia was malignancy, its correction would not occur until the underlying cause, which is seminoma, would be treated. They were, therefore, started on carboplatin and etoposide without delay, along with anti-hypercalcaemic measures. This report also highlights the value of ‘pre-phase’ chemotherapy with carboplatin, which is considered an inferior drug compared to cisplatin in the chemotherapy of germ cell tumours. In a hypercalcaemic state, renal insufficiency can be caused by pre-renal involvement, direct modifications in intravascular tone, and glomerular permeability. According to a study, renal function improved in all instances when serum calcium levels dropped with the treatment of hypercalcaemia.11 The approach to hypercalcaemia management depends on the severity of hypercalcaemia. Patients with severe hypercalcaemia are primarily managed with intravenous hydration, along with calcitonin to prevent calcium resorption from the bone.3
In the authors’ patient, furosemide was used to increase urine output, prevent fluid overload, and enhance calcium excretion. Bisphosphonates such as zoledronic acid are the treatment of choice for treating paraneoplastic hypercalcaemia. Since it takes 2–3 days to begin its action, its use is not ideal in an emergency,12 and since nephrotoxicity hinders its use in renal failure,3 it was avoided in the authors’ patient. Unlike bisphosphonates, calcitonin exhibits quick onset of action and is, therefore, utilised for an emergency.13 However, its clinical value is limited because of downregulation of calcitonin receptors resulting in tachyphylaxis within 48 hours of its initiation. The addition of glucocorticoids leads to upregulation of calcitonin receptor and boost the impact of calcitonin.14,15 To aid patients who have failed to respond to bisphosphonates or who are unable to take bisphosphonates due to severe renal impairment, denosumab is the new option.16
CONCLUSION
In summary, paraneoplastic hypercalcaemia is a rare scenario in classical seminoma. Pre-phase chemotherapy with carboplatin and etoposide to treat the underlying cause of hypercalcaemia concurrently with aggressive hydration, calcitonin, bisphosphonates/denosumab, diuretics, and dexamethasone aided in resolving hypercalcaemia, as well as restoration of normal renal function in the authors’ patient. This case report signifies the importance of treating the principal cause of hypercalcaemia along with the value of pre-phase chemotherapy in the setting of acute renal impairment.







