Abstract
Background: Kidney cancer accounts for 2.6% of all visceral malignancies in the USA. Around 5–10% of patients with renal cell carcinoma (RCC) have renal venous involvement. Open nephrectomy with tumour thrombectomy has classically been the gold standard for treatment of these masses. As opposed to open surgery, minimally invasive surgery is associated with less intraoperative blood loss, shorter hospital stays, and lower complication rates. In this study, the authors present a series of robotic radical nephrectomies in patients with renal venous invasion.
Materials and methods: Between November 2016 and March 2021, 10 patients with RCC with renal venous invasion underwent radical nephrectomies. In eight patients, renal venous invasion was evident based on CT. In four cases, tumour thrombus invaded the inferior vena cava. In three of these cases, the tumour thrombus was able to be milked back into the renal vein, allowing for ligation and transection in the standard fashion. In the remaining case, cavotomy and tumour thrombus extraction was required.
Results: All cases were performed completely robotically, without requiring open conversion. Median operative time was 136 minutes. Median estimated blood loss was 450 mL. Median length of hospitalisation was 2.5 days. Eight patients had no complications following the procedure.
Conclusion: In the setting of a community hospital, robotic management of patients with T3a and T3b RCC with venous invasion is a safe and effective alternative to open surgery.
Key Points
1. Minimal invasive renal surgery has advanced considerably over the last decade and has demonstrated several benefits such as intra-operative blood loss, decreased length of stay, and reduced post-operative analgesia requirements.
2. In this case report series, the authors perform radical nephrectomies in 10 patients with either T3a or T3b renal cell carcinoma and renal venous invasion in a community hospital.
3. Open surgery presents several complications including longer hospital stays; however, robotic management of renal tumours with venous invasion can be performed safely and effectively.
INTRODUCTION
Kidney cancer accounts for 2.6% of all visceral malignancies in the USA, with renal cell carcinoma (RCC) comprising the vast majority.1 Classic presentation of RCC includes the triad of flank pain, haematuria, and a palpable abdominal mass. However, it is now more common for RCC to be discovered incidentally on imaging or following the workup for haematuria. Risk factors include smoking, obesity, hypertension, chronic kidney disease, and inherited disorders such as Von Hippel–Lindau or polycystic kidney disease.2 Biopsy of lesions found on CT suspicious for kidney cancer is controversial, and masses are often definitively managed with surgical removal.3 As the disease progresses, the renal venous system can be compromised by the tumour and growth may continue to the inferior vena cava (IVC) and right atrium. Pathologic T3a+ disease with renal vein involvement is seen in 4–10% of all patients with RCC.1 The gold standard treatment for T3a+ disease is nephrectomy and tumour thrombectomy.4 Unfortunately, the 5-year cancer-specific survival rate is only 25–53%, which portends a worse prognosis than localised RCC.1 Conventionally, open nephrectomy with tumour thrombectomy has been the standard of care for treatment of masses with venous invasion. However, it has become increasingly common for these masses to be treated with minimally invasive surgery (MIS).4 MIS has been shown to have less intraoperative blood loss, shorter hospital admissions, improved time to convalescence, and lower complication rates than open surgery.5 While many previous studies detailed the use of MIS at tertiary academic centres, the goal of this case series is to demonstrate robotic management of RCC with renal vein or IVC involvement at a community hospital.
MATERIALS AND METHODS
Following institutional review board approval, this study represents a monocentric, retrospective review of robotic assisted nephrectomies with or without IVC thrombectomy. All operations were performed by a single high-volume robotic surgeon (Maatman) from November 2016 to March 2021. A total of 59 cases were completed by the primary surgeon between this time. Thirty-seven patients were selected who were at least 18 years or older and had final pathologic staging of T3a or greater. Of these patients, only 10 patients had venous invasion and were included for final analysis. The remaining 27 were excluded because they had either perinephric or collecting system invasion without venous involvement on final pathology. All patients included in the study had CT imaging obtained perioperatively.
Data collected included patient demographics (age, laterality, comorbid factors, BMI, and American Society of Anesthesiology [ASA] score); intraoperative estimated blood loss, operative time, and postoperative factors (length of stay, postoperative complications) as well as final pathology. Preoperative imaging was also reviewed by institutional board-certified radiologists to evaluate for gross involvement of the renal venous system. A complete breakdown of patient demographics is demonstrated in Table 1.
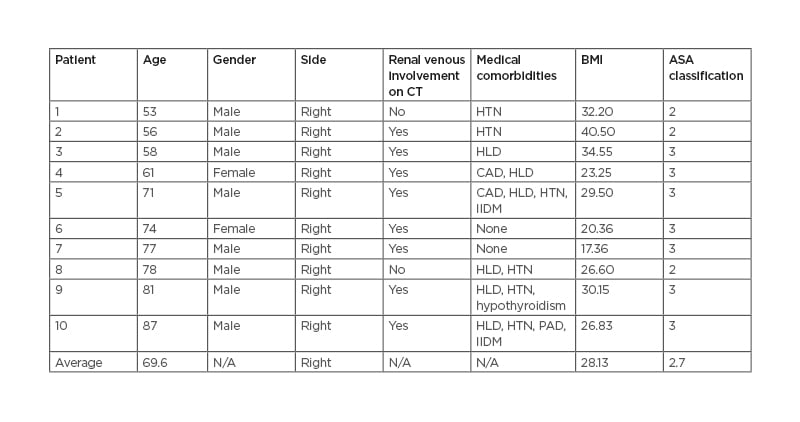
Table 1: Patient demographics and preoperative characteristics.
ASA: American Society of Anesthesiologists; CAD: coronary artery disease; HLD: hyperlipidaemia; HTN: hypertension; IIDM: insulin-independent diabetes mellitus; N/A: not applicable; PAD: peripheral artery disease.
SURGICAL TECHNIQUE
The da Vinci Si and Xi Surgical Systems (Intuitive, Sunnyvale, California, USA) were used for all cases. Each case was started by medialising the colon and exposing the renal hilum. In almost every case a gross tumour thrombus was visible in the renal vein. The renal artery was then exposed, ligated, and transected. After transection of the renal artery, the treatment algorithm would diverge based on the involvement of the tumour thrombus. In those with tumour thrombus extending only into the renal vein, a robotic stapler was used to ligate the vein after ensuring mobility of the tumour thrombus and ability to maintain a satisfactory margin (Figure 1).
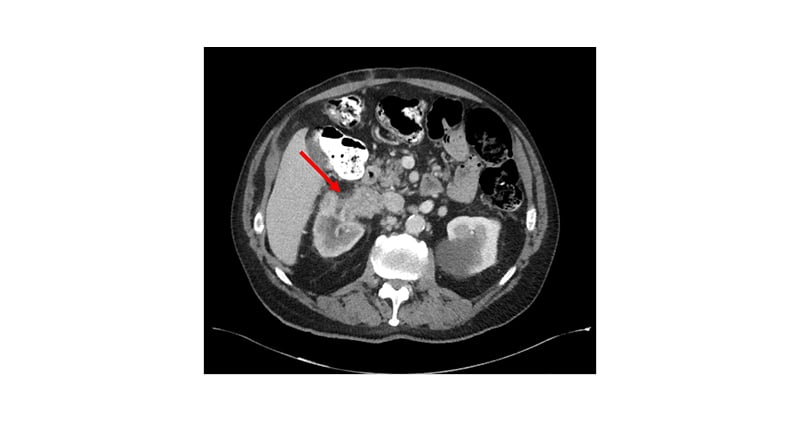
Figure 1: Axial CT imaging of a patient’s abdomen, demonstrating tumour thrombus invading the right renal vein and inferior vena cava.
Tumour thrombosis measured 10x8x4.5 cm.
Thrombus Extension That Does Not Require Cavotomy
When gross tumour thrombus was seen extending into the IVC, dissection was performed down to the level of the iliac bifurcation. A vessel loop was then wrapped doubly around the IVC below the level of the thrombus. The IVC superior to the thrombus and the left renal vein were mobilised and vessel loops were placed around these vessels in a similar fashion. Following placement, the vessel loops were pulled through 24 Fr Foley catheter rubber shods. In the event vascular occlusion was needed, the Rummel tourniquets would be able to be cinched down and secured with Hem-o-lok clips (Teleflex®, Wayne, Pennsylvania, USA). In almost all of these patients, occlusion was not needed, and the tumour thrombus was able to be pressed into the renal vein by serially grasping the proximal edge of the thrombus.
Thrombus Extension Requiring Cavotomy
In one of the patients, the thrombus was unable to be mobilised back into the renal vein and access into the lumen of the IVC was deemed necessary. The Rummel tourniquets were then used to secure the vasculature as previously described. Next, the IVC was sharply dissected open, and the tumour thrombus was extracted. The IVC was closed with running 3–0 silk suture. The tourniquets were released, haemostasis was confirmed. The kidney was then dissected free, and the procedure was completed in the standard fashion.
RESULTS
All cases were successfully performed robotically without conversion to open. Mean patient BMI was 28.2 (range: 17.4–40.5) and mean ASA score was 2.7 (range: 2–3). All additional preoperative patient characteristics are listed in Table 1. Of note, all patients had right sided disease.
In eight cases, gross tumour thrombi were visibly extending into the renal vasculature on CT (Figure 1). In two cases, the preoperative imaging was not suggestive of thrombus and the venous extension was discovered at the time of pathologic evaluation. Surgical margins were negative in eight patients and positive in two. The two patients with positive margins had T4 disease radiographically. For one of these patients, the procedure was performed for palliative purposes for multiple life-threatening episodes of haematuria. The second patient with T4 disease and a positive margin had a cytoreductive nephrectomy to prepare for systemic chemotherapy.
Eight patients had no complications according to the Clavien–Dindo classification. One patient had a Clavien Grade II for acute blood loss anaemia, requiring blood transfusion. Another patient had a Clavien Grade IIIa as they required readmission and nasogastric tube placement for a postoperative ileus. Nine of the 10 tumours were clear cell RCC and one was papillary RCC. The median blood loss was 450 mL (60–900), the median length of inpatient stay was 2.5 days (1–4), and the median operative time was 136 minutes (97–191 [hl]Table 2[/hl]). In three cases, the tumour thrombus was able to be milked back into the renal vein from the IVC prior to ligation. In one case tumour thrombus extension into the IVC required cavotomy as the thrombus was unable to mobilised.
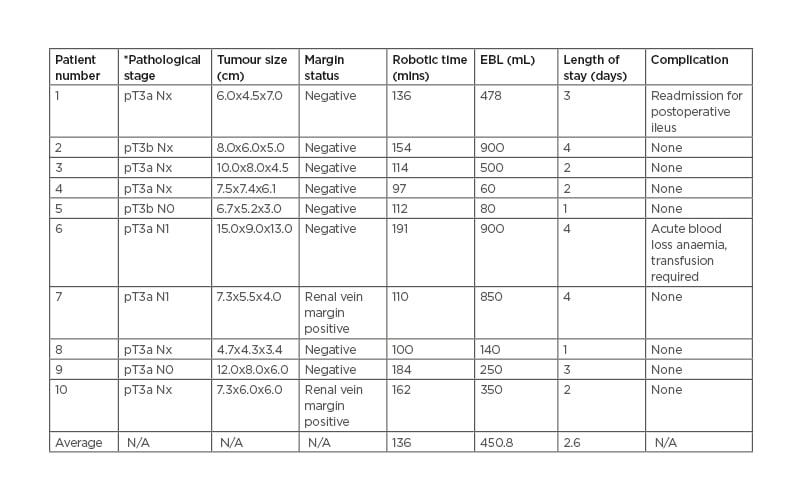
Table 2: Postoperative outcomes.
*Pathological staging based on the American Joint Committee on Cancer (AJCC) classification.
EBL: estimated blood loss; pT: pathologic tumour; N: nodes; N/A: not applicable.
DISCUSSION
The first laparoscopic radical nephrectomy was performed in 1990 on an 85-year-old female with a 3 cm mass. It took 7 hours, and the patient was discharged on post-operative Day 6.6 Since then, minimally invasive renal surgery has advanced considerably, and the approach has been widely adopted. Significant benefits over open surgery have been demonstrated including less intraoperative blood loss, decreased length of stay, and reduced postoperative analgesia requirements.6 The utilisation of the laparoscopic approach peaked in 2008 when it neared 50% of all cases. In 2004 the first report of a robot assisted radical nephrectomy was published, and this approach continues to increase in popularity. As many as 27% of all radical nephrectomies were performed robotically in 2015.7 Some argue that a purely laparoscopic approach is the optimal method to perform a radical nephrectomy as the robotic approach is more expensive and does not have significantly improved outcomes.7 At the authors’ institution, they prefer to use the robotic approach as it allows the trainees to hone their skills for other robotic procedures such as partial nephrectomies, nephroureterectomies, and pyeloplasties.
Analysis of the literature demonstrates that perioperative outcomes related to RCC with IVC thrombus are often associated with complications. In one study of 5,180 patients from 416 institutions, 4% of patients had a major postoperative complication, 28% had a complication of any kind, more than 40% of patients had an operative time of greater than 4 hours, 20% of patients required a blood transfusion, and 20% of patients required hospitalisation for more than 4 days.8 In this study’s series, only one of the patients required transfusion (12.5%), operative time never exceeded 4 hours, and length of stay never exceeded 4 days.7 All of the tumours in this series except one were at least 7 cm in diameter and almost all had gross extension of tumour thrombus. Even in those with large tumour burdens, this series confirms safety and efficacy in the treatment of these patients in a community setting.
In two patients, renal venous invasion was discovered during pathologic processing. As a result of this finding, both of their T stages were upgraded from T1 to T3. In one study of 987 patients with cT1a tumours, 9% were upstaged to pT3a as a result of renal venous invasion on pathology. These patients were found to have lower 2-year recurrence free survival (87.3%) than those that were not upstaged (98.7%).8 This finding was redemonstrated in a study of almost 2,000 patients at the University of Michigan, USA, in 2018. Russell et al.9 found both the 3-year and 5-year recurrence free survival of upstaged tumours to be significantly lower than those of their matched non-upstaged cohort members. The difference in 5-year progression free survival was particularly drastic with the non-upstaged progression free survival rate of 96% compared to the upstaged progression free survival rate of 76%.9
In the staging of RCC, the grade of T3a can be quite variable. The size of the tumour is no longer a factor, and the primary determinant of staging is the degree of extra renal extension. Extra renal extension can occur in multiple places including renal sinus fat, renal venous system, or perirenal fat. As these tumours can have various sizes and types of invasion, their prognosis can vary significantly. Renal venous thrombosis (RVT) is a particularly poor prognostic factor and is a predictive factor for postoperative recurrence in patients with T3. One study of 800 patients found that those with clinically detected RVT had a 5-year recurrence free survival rate of 24.9% as opposed to 74.7% in other patients with T3a disease.8 They also found that those with RVT had a 5-year cancer specific survival rate of 70.5% compared with 92.6%.8
The study represents a community-based, single institution’s results, and operative techniques in those undergoing radical nephrectomy with renal venous invasion. Primary limitations of this study include its retrospective nature, only single surgeon performing all the cases, and the small sample size. The sample size is limited as RCC with significant venous invasion is relatively rare. Selection bias is an additional limitation as those with T3 disease that were poor candidates for surgery were not operated on.
CONCLUSION
Traditionally, renal tumours with gross tumour thrombi have been treated with open surgery, especially outside of the setting of a large academic hospital. Open surgery is associated with longer hospital stays, more postoperative pain, greater blood loss, and higher complication rates. This case series demonstrates that the robotic management of renal tumours with venous invasion can be performed safely and effectively in a community setting.








