Abstract
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a debilitating condition affecting approximately 3% of the female population. IC/BPS is defined as an unpleasant sensation (pain, pressure, discomfort) perceived to be related to the urinary bladder, associated with lower urinary tract symptoms for more than six weeks duration, in the absence of infection or other identifiable cause. This condition is known to have a profound negative impact on quality of life. There are few well-studied treatment options and no cure for this condition, which is therefore challenging to treat. The purpose of this narrative review is to summarise the contemporary literature, including the Canadian Urological Association (CUA) and American Urological Association (AUA) guidelines, on various treatment options that exist for IC/BPS, including conservative therapies, oral therapies, intravesical therapies, and more invasive surgical options. Most importantly, this review highlights the need for an individualised, multimodal approach to the treatment of IC/BPS.
INTRODUCTION
Interstitial cystitis/bladder pain syndrome (IC/BPS), is among one of the most common diagnoses that give rise to chronic pelvic pain.1 Currently, the definition referred to by the Canadian and American Urological Associations (CUA and AUA), as well as the European Association of Urology (EAU), is one offered by the Society for Urodynamics and Female Pelvic Medicine and Urogenital Reconstruction (SUFU). They define IC/BPS as “an unpleasant sensation (pain, pressure, discomfort) perceived to be related to the urinary bladder, associated with lower urinary tract symptoms for more than 6 weeks duration, in the absence of infection or other identifiable causes.”2 The duration of pain or discomfort required for diagnosis has varied across definitions from 4 weeks to 6 months. A shorter required duration of pain is thought to facilitate earlier treatment.3 IC/BPS is considered a diagnosis of exclusion, confirmed after the exclusion of other urological and gynaecological conditions such as urinary tract infection, malignancy, overactive bladder, and endometriosis.
Because of the progression in its definition, the epidemiology of IC/BPS has been difficult to determine. American data suggest a prevalence of between 2.7% and 6.5% of females.4 IC/BPS affects both females and males, though studies have shown a 5:1 female-to-male preponderance.5 However, it is thought that there is a dramatic under-reporting of this condition in men attributable to the significant symptom overlap of chronic prostatitis/chronic pelvic pain syndrome.6,7
The aetiology of IC/BPS is poorly understood. Multiple theories exist, including disruption in the permeability of the urothelium lining the bladder (glycosaminoglycan deficiency), infection, autoimmune activation, mast cell infiltration, and neurogenic mechanisms. Approximately 5–10% of patients presenting with this symptom complex will be found to have ulcerations in the bladder known as Hunner’s lesions (HL).8 HL are associated with more severe symptoms and decreased bladder capacity, although it is not possible to identify patients with HL based on symptoms alone. Recommendations on the utility of cystoscopy vary between guidelines.3The CUA guidelines list cystoscopy as ‘recommended’ for all patients with IC/BPS, while the AUA guidelines consider cystoscopy ‘optional’.1 The aetiology of HL is yet to be determined. Biopsy of these lesions is mandatory to rule out other underlying disorders such as carcinoma in situ, urothelial carcinoma, other malignancy, nephrogenic adenoma, or eosinophilic cystitis.
Treatment must be focussed on maximising quality of life (QoL), as there is no treatment that will change the natural history of this condition or cure IC/BPS. Traditionally, the treatment of IC/BPS has been approached in an algorithmic fashion; however, there has been a shift towards treating patients based on symptom phenotypes.1,6 A classification system, UPOINT, has been proposed as a way to direct multimodal therapy with an individualised approach. UPOINT domains include urinary, psychological, organ-specific, infection, neurologic, and muscle tenderness.9
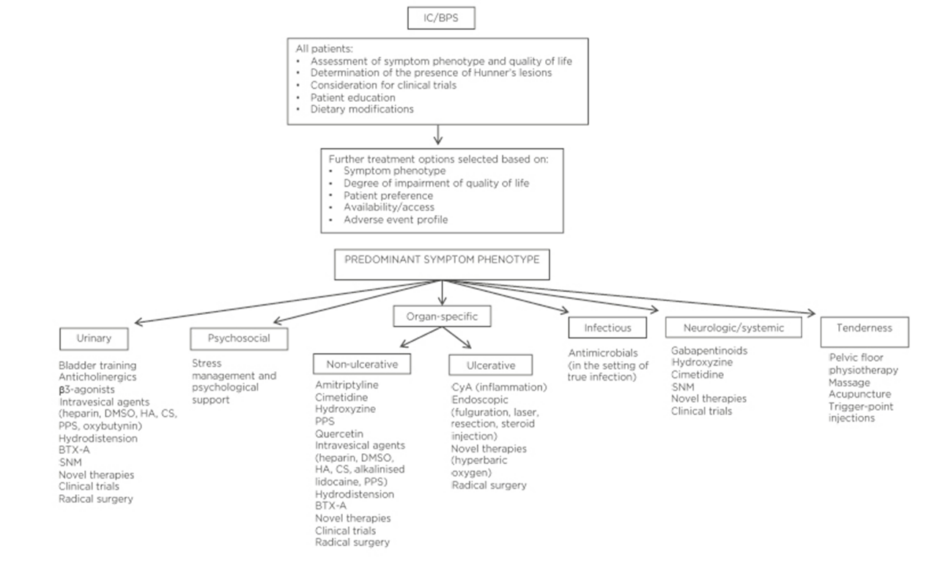
In addition to symptom phenotype, treatment options should be based on the degree of QoL impairment, patient preference, availability or access, and adverse event profile. Figure 1 is adapted from the CUA guidelines on IC/BPS and offers a summary for the management options for IC/BPS.6
IC/BPS, whether nonulcerative or ulcerative, remains a poorly understood entity which lacks effective, evidence-based treatments. IC/BPS presents healthcare professionals with a dilemma when selecting appropriate treatment options for patients; multiple treatments already exist but the evidence supporting these treatments is often conflicting. In addition, new treatments covering a variety of therapeutic mechanisms are continuously being investigated. Decisions regarding treatment selection, and keeping up-to-date on new treatments being explored, remain a challenge for healthcare professionals. The rationale for this narrative review is to offer clinicians a practical approach to the treatment of both nonulcerative (NUIC) and ulcerative IC/BPS (UIC) and secondly, to provide a brief update on potential novel therapies for the treatment of IC/BPS
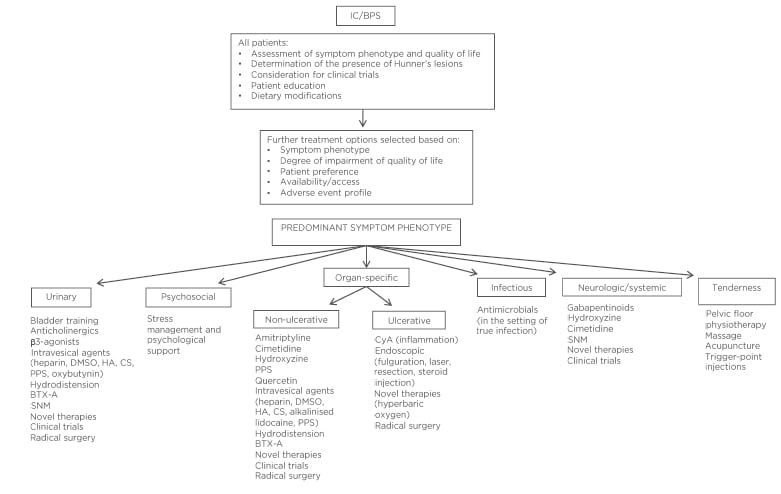
Figure 1: Proposed management paradigm for the treatment of interstitial cystitis/bladder pain syndrome.
BTX-A: OnabotulinumtoxinA; CS: chondroitin sulfate; CyA: cyclosporine A; DMSO: dimethyl sulfoxide; HA: hyaluronic acid; IC/BPS: interstitial cystitis/bladder pain syndrome; PPS: pentosan polysulfate; SNM: sacral neuromodulation.
Adapted, with permission, from the Canadian Urological Association (CUA) Guidelines.6
METHODS
A literature search was carried out for this narrative review. PubMed was searched using the terms ‘interstitial cystitis’ and ‘bladder pain syndrome’ over the last 5 years. The authors searched using the filters “English”, “core clinical journals”, “age 19+” and “humans”. In addition, recent guidelines from major urological associations, as well as other review articles, were examined to obtain pertinent references on standard recommended treatment options for IC/BPS and novel therapies.
NONULCERATIVE DISEASE
Conservative Therapies
Therapies recommended to all patients with IC/BPS, regardless of subtype, include education, dietary modifications, bladder training, and adaption of stress-management techniques. There is substantial evidence to suggest that 40–50% of patients will improve with adherence to these principles alone.10,11 Up to 90% of patients report dietary triggers for their IC/BPS symptoms.12 Common dietary triggers include acidic foods (i.e., tomatoes, citrus), caffeine, alcohol, spicy foods, and artificial sweeteners. Bladder training, including urge-suppression and distraction techniques, may be beneficial.13 Psychological stress is known to be a trigger for IC/BPS symptoms and techniques including yoga, reduced work hours, and exercise are thought to be beneficial.14
Up to 87% of patients with IC/BPS will have concomitant pelvic floor muscle dysfunction and muscle tenderness.15 These patients may also report issues with dyspareunia and bowel dysfunction. These patients have a high chance of symptom improvement and a 21% chance of cure with pelvic floor physiotherapy.16,17Massage techniques and physiotherapy are recommended by the CUA for these patients. These techniques may be costly and difficult to access for some patients.
Medical Management
There are a variety of oral therapies that have been studied for the treatment of IC/BPS, including amitriptyline, pentosan polysulfate (PPS), hydroxyzine, cimetidine, gabapentinoids, and cyclosporine A (CyA). In general, medications are reserved for patients who have failed a trial of conservative therapies alone.
PPS (Elmron®) is the only oral medication approved by the U.S. Food and Drug Administration (FDA) and Health Canada for the treatment of IC/BPS. PPS is an oral heparinoid and is thought to exert its effect through replacement of the urothelial glycosaminoglycan layer.18 Multiple small, placebo-controlled, randomised controlled trials (RCT) have been completed, reporting conflicting results, possibly because of the study design. A meta-analysis, including data on >400 patients, summarised these results comparing PPS to placebo; the meta-analysis concluded a significant improvement in symptoms of pain (37%), urgency (28%), and frequency (54%), but not nocturia.19 There is an ongoing observational trial assessing the outcomes of PPS in combination with hydrodistension.20 The therapeutic effects of PPS may not be seen for up to 6 months. PPS may also be administered intravesically.21 PPS is costly and may result in side effects, including diarrhoea, nausea, headache, abdominal pain, and reversible alopecia. In addition, a recent association between long-term PPS use (median 19.2 years) and vision-threatening maculopathy has been reported.22 Given the significant improvements in up to 44% of patients and the low rate of serious adverse events, PPS has been considered an option in the CUA and AUA guidelines.1,6
The tricyclic antidepressant amitriptyline has anticholinergic, antihistamine, analgesic, and sedative properties. There is evidence to support the use of amitriptyline, as placebo-controlled RCT have found a statistically significant improvement in symptom scores (p=0.005), and urinary urgency and pain (p<0.001) in comparison to placebo.23,24 Trials fail to show a benefit in doses <50 mg and side effects at this dose are common.10 Side effects include sedation, dry mouth, and constipation. Amitriptyline is considered an option by the CUA and AUA, after conservative strategies alone have failed.
Excess production of mast cells within the detrusor muscle of the bladder, leading to histamine release, is one of the proposed causes of IC/BPS. Following this theory, the use of cimetidine, an H2-histamine antagonist, has been investigated for the treatment of IC/BPS. Its use is supported by evidence from two small, observational trials and a small RCT, with no reported side effects.25 Symptoms of nocturia and suprapubic pain were among those most improved. Current regimes practised are 400–800 mg/day divided between two or three doses.
Hydroxyzine, another antihistamine, has been studied in a few small RCT, with conflicting results. One observational study found a 40% reduction in symptoms. In another RCT, there was minimal benefit of hydroxyzine versus placebo; however, there was a benefit with adding hydroxyzine to PPS, increasing the response rate to 40% versus 28% with PPS alone.26 Side effects of hydroxyzine are common and may include drowsiness, constipation, dry mouth, and gastrointestinal symptoms.
Many other oral agents have been studied for treatment of IC/BPS, with very limited or conflicting results. Not all of these medications have been included in guideline statements but may be of value when treating this patient population. Patients should be informed of the lack of large trials to support the use of these medications for IC/BPS and be made aware of potential side effects. These agents include gabapentin, pregabalin, quercetin, montelukast, sildenafil, and L-arginine.27
Intravesical Therapy
Several agents have been studied for intravesical use in the treatment of IC/BPS. Examples of these include heparin, dimethyl sulfoxide (DMSO), multi-agent combinations, PPS, hyaluronic acid, chondroitin sulfate, lidocaine, bacillus Calmette–Guérin (BCG), and resiniferatoxin. There is no consensus on the frequency, duration, or dose of intravesical therapies for IC/BPS. Common side effects of intravesical therapies include mild discomfort, haematuria, and urinary tract infection. Based on evidence showing no convincing improvement in symptoms and a high side effect profile, BCG and resiniferatoxin should not be used.
DMSO is the only FDA- and Health Canada-approved intravesical agent. Its mechanism of action is thought to include anti-inflammatory and muscle relaxant effects. Data on the effectiveness of DMSO seem to be dated and conflicting. A 2007 Cochrane review reported no significant improvement over placebo;28however, further studies suggested there may be a role for DMSO, particularly in patients with ulcerative disease.29 Treatment with DMSO may cause halitosis (‘garlic breath’ odour) or temporary flare of symptoms after the initial treatment.
Heparin is thought to exert anti-inflammatory and angiogenesis-promoting effects on the bladder mucosa. It may be used alone (20,000–40,000 units diluted in 10–50 mL of normal saline) or in conjunction with lidocaine, sodium bicarbonate, and DMSO.6 Several studies have suggested a symptomatic improvement with heparin at a variety of doses (56–73% of patients with symptomatic improvement at 3 months).30,31Heparin instillations may be administered by the patient at home on an as-needed basis and represent an option for the treatment of symptomatic flares. Further research to confirm the benefit of the abovementioned intravesical therapies would be beneficial but novel, large, well-designed trials are lacking. Despite this, these therapies are used commonly. Intravesical therapies remain second-line therapies based on the AUA guidelines and are recommended in select patients based on the CUA guidelines.
Hydrodistension (Bladder Dilation)
Despite a lack of standardised technique, hydrodistension has been used for almost 100 years and is one of the most commonly used treatments for IC/BPS.32 This technique involves performing a cystoscopic examination under general anaesthetic and filling the bladder with sterile water to its maximum anaesthetic capacity at a pressure of 80–100 cm H2O. Theoretically, it is beneficial due to a temporary ischaemia to nerve endings resulting in a decrease in bladder pain and increased bladder capacity. There is a lack of randomised data for this technique.33 Dated observational studies report variable findings, with a response rate ranging from 30–54% at 1 month to 0–37% at 6 months following treatment.6 The treatment effects are not permanent and the procedure may need to be repeated. Data regarding the long-term effects of repeat hydrodistension are lacking. A contemporary systematic review evaluated the evidence for the use of hydrodistension for BPS, focussing on patient-related outcomes. Seventeen studies were included, none of which used a validated outcome measure to assess the effect of hydrodistension alone.33 The AUA guidelines suggest hydrodistension be used as a third-line therapy and it is considered optional in select patients by the CUA.
OnabotulinumtoxinA (BTX-A)
OnabotulinumtoxinA (BTX-A) has been studied for the treatment of IC/BPS based on the antinociceptive and motor-paralytic actions of this agent. It is approved for the treatment of overactive bladder and urgency incontinence and is widely used. Multiple small RCT have been conducted on patients with IC/BPS with conflicting results. A contemporary meta-analysis of 12 RCT, including 459 patients, found a significant improvement in Interstitial Cystitis Symptom Index (ICSI) and Problem Index (ICPI) scores, pain scores, and daytime frequency in patients with IC/BPS treated with BTX-A.34 A recent network meta-analysis of intravesical therapies for IC/BPS found that BTX-A, in comparison to instilled intravesical therapies, resulted in the greatest improvement based on global response assessment (GRA).35 Larger RCT are required to confirm these results. Several variations to the delivery of intravesical BTX-A have been studied, including injecting BTX-A into the bladder trigone as opposed to the posterior bladder wall, and instillation of BTX-A into the bladder in a liposomal formulation.36,37 Patients must be aware of the potential risk of urinary tract infection, haematuria, and need for temporary clean intermittent catheterisation following BTX-A. BTX-A is also an option for patients with UIC. BTX-A is considered a fourth-line therapy for IC/BPS based on the AUA guidelines and an optional treatment based on CUA guidelines.
Sacral Neuromodulation
Sacral neuromodulation (SNM) is not approved for the treatment of IC/BPS but is used for urgency incontinence and frequency–urgency syndrome, both of which commonly occur with IC/BPS. SNM involves the implantation of a permanent tined lead into the third sacral foramina to regulate the afferent sacral nerve and modify bladder function. Multiple observational trials demonstrate a 42–95% improvement in symptoms.38,39 Peters et al.38 demonstrated a decrease in narcotic use following SNM implant. Long-term success rates approach 72% up to 62 months.40 To date, RCT are lacking. Patients must be considered appropriate surgical candidates and be aware of the risks, including failure, need for surgical revisions, pain, and infection. The surgical revision rate for reasons other than routine battery change ranges from 27–50%. The AUA considers SNM to be a fourth-line therapy, while the CUA considers SNM optional in select patients. This technology may not be widely available at all centres.
ULCERATIVE DISEASE
UIC is characterised by the presence of ulcer-like lesions in the bladder lining. HL are identified on cystoscopic evaluation. The prevalence of HL ranges from 5–10%.41,42 There is some evidence to suggest that the pathophysiology of pain associated with UIC differs from that of NUIC.41 In addition to the therapies for NUIC, treatment with immunomodulating agents such as cyclosporine A, fulguration of ulcers, and major surgery in the form of urinary diversion are additional options for the treatment of UIC.
Cyclosporine A
CyA is an immunosuppressive agent involved in the regulation of T cells. As autoimmune dysfunction has been suggested as a potential cause of UIC, CyA has been studied for the treatment of IC/BPS at varying doses. RCT have compared CyA in addition to PPS versus PPS alone and found a superior effect with the addition of CyA (59% versus 13%; p<0.001).43 Patients with HL appear to derive more benefit from CyA than those without ulcers (68% versus 30% response rate, respectively).44 More recently, a systematic review assessed the treatment effect of CyA in patients with IC/BPS. Eight studies were included, three of which were RCT. The authors concluded that treatment with CyA could potentially result in long-term benefit; however, further evidence is required to confirm these findings.45 CyA may be dosed at 2 mg/kg divided into twice daily (bid) dosing and drug levels should be monitored. Side effects are common, and patients must be monitored for renal impairment, hepatic impairment, electrolyte abnormalities, hypertension, and infection. Frequent side effects and the need for strict monitoring while on therapy has limited the use of CyA in clinical practice. However, Crescenze et al.46 reported novel data on a large cohort of patients with UIC, of which 47% (26/55) were treated with CyA with favourable results. The AUA guidelines consider CyA a fifth-line therapy, while the CUA guidelines list CyA as an option in patients refractory to other therapies. CyA may be an appropriate therapy at centres with prior experience and supports in place to facilitate patient monitoring.
Ulcer Fulguration
Primary endoscopic ablation of HL has been used since 1971.47 Treatment of HL has been shown to significantly decrease urinary symptoms including daytime frequency, urgency, and nocturia.48 In a large observational study, 90% of 103 patients reported symptomatic relief, which lasted for up to 3 years in 40%.49 Electrocautery fulguration, laser ablation, or resection of HL are accepted and recommended treatment options.1,46 When comparing transurethral resection of HL to transurethral coagulation, Ko et al.50found no significant difference in HL recurrence-free time (12.2 versus 11.5 months for transurethral resection versus coagulation; p=0.735). There was also no difference in symptomatic improvement between groups, but the transurethral resection group had an increased rate of bladder injury compared to transurethral coagulation (7.9% versus 3.4%).50 Although initial treatment of lesions often results in symptomatic relief, ulcers recur and require retreatment in the majority of patients.
Intralesion Triamcinolone Injection
Triamcinolone is a long-acting synthetic steroid. Central injection of triamcinolone into HL at a depth of 2–3 mm has shown therapeutic benefit and is a therapeutic option for UIC.51,52 Observational studies report a 70–91% response rate that lasted between 7 and 12 months.46,52 Repeat injections are likely to be required and thought to be safe. Dosing consists of 1 mL vial of triamcinolone (40 mg/mL) diluted in 9 cm3 of injectable normal saline, which can then be injected in 1 cm3 aliquots.7 This outpatient procedure is done through a cystoscope under general or spinal anaesthetic.
Invasive Surgical Procedures
Radical surgery in the form of urinary diversion is considered as a last resort for severe UIC. Urinary diversion may be performed with or without a concomitant cystectomy and is usually in the form of an ileal conduit. Historically, augmentation cystoplasty was performed. There are several case reports evaluating patient outcomes following urinary diversion. Andersen et al.53 reported a 74% pain-free rate and 68% satisfaction rate following surgery. Of those who do not have a cystectomy performed at the original surgery, 17–22% will go on to require a cystectomy after urinary diversion because of ongoing pain.53,54 Patients with identifiable disease (i.e., HL) in the bladder and those with diminished maximum anaesthetic bladder capacity based on findings at hydrodistension are more likely to find an improvement in pain and urinary symptoms following urinary diversion. The complication rate is high with this type of surgery and ranges in severity. According to the AUA and CUA guidelines, radical surgery should be considered an absolute last resort for patients with IC/BPS and reserved for patients with severe UIC.
NOVEL TREATMENTS
Novel treatments are constantly being investigated for the treatment of IC/BPS. Examples include oral therapies, hyperbaric oxygen, extracorporeal shockwave lithotripsy, stem cell therapy, and cannabinoids. Several oral therapies are being investigated for future use in the treatment of IC/BPS. Neurotropic growth factors, such as nerve growth factor (NGF), are upregulated in inflammatory conditions including IC/BPS.55Monoclonal antibodies directed against NGF have been under investigation including tanezumab and fulranumab; these studies have been terminated because of the adverse side-effect profile. To avoid systemic effects of this medication class, the use of a liposomal NGF delivery system is being investigated.55
Rosiptor (AQX-1125) is an oral SHIP-1 activator that is thought to negatively regulate the PI3K pathway to reduce an immunological reaction, acting as an anti-inflammatory. Tipelukast (MN-001) is an oral agent that inhibits phosphodiesterases and acts as a leukotriene receptor antagonist, also exerting an anti-inflammatory effect. Suplatast tosilate (IPD-1151 T) is another novel agent under investigation, based on its immunomodulating effects suppressing IgE. These, and several other agents, represent potential future treatments for IC/BPS. Recently, however, Nickel et al.56 reported negative results from a 12-week randomised, double-blind, placebo-controlled, Phase 3 clinical trial showing no significant difference in daily bladder pain in patients treated with AQX-1125 compared to placebo. This study also highlighted the challenges of designing clinical trials for future intervention strategies for IC/BPS.
The use of hyperbaric oxygen chambers has been suggested to improve IC/BPS pain intensity by nearly 30%, with a 15% increase in single-void volumes.57 There is some evidence to suggest that hyperbaric oxygen may be more effective for UIC compared to NUIC.58 The effect appears to be related to enhanced O2delivery to the bladder mucosa during the filling phase, the phase when the majority of patients experience symptoms.
Very recently, a prospective, multicentre, double-blind RCT reported a decrease in pelvic pain scores following pelvic extracorporeal shockwave lithotripsy (N=24; 2,000 shocks; 3 Hz; maximum total energy flow density: 0.25 mL/mm2) versus a placebo treatment (N=25; shockwave setting without energy transmission); however, the study did not meet the primary endpoint of change in O’Leary-Sant symptom scores. No significant adverse events were detected.59
Based on a similar mode of action as CyA, intravesical tacrolimus has been investigated as a treatment for IC/BPS. In a recent pilot study, Mishra et al.60 found that 54% of patients (13/24) treated with intravesical tacrolimus (0.1 mg/kg) dissolved in DMSO/sterile water reported a significant improvement in symptoms based on a global response assessment (GRA). Minimal side effects were noted and it was well tolerated.
The use of stem cells for IC/BPS has not been investigated in human subjects to date. However, stem cells have proven to be a viable option in animal models of IC/BPS.61 Future study is directed towards introducing stem cells into trials on human subjects for various urological conditions.
Cannabinoid (CB) receptors are being investigated because of their potential for anti-inflammatory and immunomodulating effects.62 Rodent models, followed by human studies, have confirmed that both CB1 and CB2 receptors are present in the urinary bladder.63 Initial rodent studies demonstrated that the activation of CB1 and/or CB2 receptors reduced bladder inflammation by blocking the peripheral mechanical sensitivity that accompanies inflammatory cystitis.64 Recently, a survey of men with chronic prostatitis/chronic pelvic pain syndrome revealed a cannabis use rate of nearly 50%. Of these, over half stated that cannabis was “somewhat/very effective” in managing their symptoms.65 The role of CB receptors in the urinary bladder remains an area of active research.66,67 To date, however, there is a paucity of clinical research investigating the use of cannabinoids in patients with IC/BPS.
CONCLUSION
IC/BPS represents a debilitating syndrome, consisting of pain perceived to be related to the bladder and associated with lower urinary tract symptoms. It has been shown to significantly impair QoL and the aetiology is poorly understood. There are numerous treatment options available but none that cure or alter the natural history of the disease. Treatments should be selected on an individualised basis, based on the primary symptomatology, patient preference, availability, and adverse side-effect profile. Differentiating NUIC from UIC is imperative as treatment of ulcers may result in a significant improvement in symptoms. A proportion of patients will require a multimodal approach to therapy and ongoing, supportive care.