Abstract
Natural killer (NK) cells are effector lymphocytes that play protective roles against both infectious pathogens and cancer. Although NK cells contribute to the innate immune system, they have a number of similarities to cells of the adaptive immune system, including T and B cells. Recent discoveries have also shown that NK cells are capable of adapting and developing into long-lived memory cells, providing new functional insights into the roles of innate immune cells. In this article, the author provides an overview of human and murine NK cell development, function, and memory, as well as their role in viral infection and cancer.
INTRODUCTION
Natural killer (NK) cells were originally described in the 1970s as large granular lymphocytes able to develop natural cytotoxicity against tumour cells without a prior encounter.1-3 They play an invaluable role in early defence against invading pathogens and cancer and are able to produce an array of cytokines and chemokines to help regulate an immune response.4 NK cells make up 5–15% of human peripheral blood and 2–3% of murine splenocytes.5 They are found in both primary and secondary immune compartments, with the majority of cells being localised in the spleen, lymph nodes, bone marrow, and peripheral blood to carry out immunosurveillance throughout the vasculature. They are also found in mucosal tissues, including the lungs, small and large intestines, and colon.6 Along with their roles in immune defence, NK cells play significant roles during pregnancy. In response to sex hormones, there is a substantial increase in uterine NK cells, which are thought to promote placental growth and provide maternal–fetal immunomodulation;7 however, both peripheral NK cells and uterine NK cells have been associated with infertility and miscarriage.8 Almost a decade ago, NK cells were recognised as a member of the lymphocyte family known as innate lymphoid cells (ILC). ILC were classified into three main groups based on their cell surface marker expression, functionality, and transcriptional regulation.9 Group 1 ILC, which originally included ILC1 and NK cells, were distinguished from other ILC groups by their constitutive expression of TBX21 and its protein product T-bet, and the production of IFN- following IL-12 stimulation.10 However, recently the ILC family has been reclassified into subsets based on their development from the common lymphoid progenitors (CLP) and their immune functions. In these subsets, NK cells are no longer grouped with ILC1.9 This article will review the development, function, and memory capacity of NK cells, along with their roles during viral infection and cancer.
NATURAL KILLER CELL DEVELOPMENT
NK cells have been classified as components of the innate immune system; however, they have also been shown to possess numerous developmental and functional characteristics similar to cells of the adaptive immune system, including T and B cells. These include the development from the CLP in the bone marrow, expression of the recombination-activating genes during ontogeny, the need for common -chain-dependent cytokines (including IL-15) during development and homeostasis, and an education process analogous to T cell development in the thymus.11,12 It has also been proposed that NK cells may develop in both the thymus and the liver.13 Furthermore, much like T and B cells, which use their activating receptors (T cell or B cell receptor, respectively) to recognise antigens, NK cells express germline-encoded activating receptors that are able to bind directly to stress-induced or pathogen-derived antigens.14
Developing from haematopoietic stem cells, CLP in murine bone marrow differentiate into pre-NK precursors with a Lin-CD117loCD127+ phenotype and express a number of NK cell-specific receptors including NKG2D and CD244,15 but during this stage the cells are negative for classical markers such as NK1.1 and CD49b. Following the expression of the -chain receptor for IL-15 (a cytokine required for NK cell development), these cells are now classed as NK precursors. Once CD122 is expressed, they become responsive to IL-15 and develop into immature NK cells, observed by CD11blo and CD27 surface expression.16 At this point, a number of activating and inhibitory receptors are also beginning to be expressed on the surface of the developing NK cells.17 CD11b and CD27 expression defines murine NK cells in four stages of maturation, which correspond with their cytolytic activity and production of inflammatory cytokines (Figure 1). During maturation, immature NK cells progress to CD11bloCD27hi, then CD11bhiCD27hi, and finally CD11bhiCD27lo.18 In humans, NK cells also develop from haematopoietic stem cells and through a CLP. During five stages of development, there are a number of changes in expression levels of CD56, CD94, and CD16, and, much like for mice, human NK cells become responsive to and require the cytokine IL-15. Maturing human NK cells can differentiate into CD56bright cells, which usually remain in the lymphoid tissue to interact with dendritic cells (DC) and CD56dim cells that return to circulation via the lymphatics.15 Human NK cells are considered fully mature when they have high cytolytic activity and are able to produce large amounts of IFN-.17
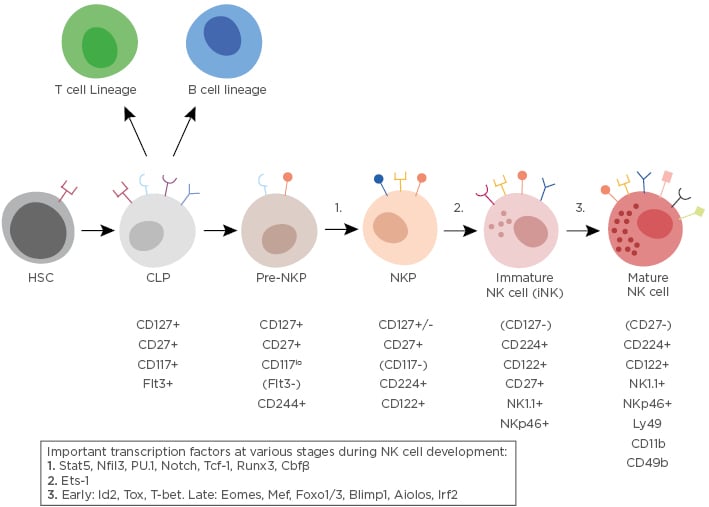
Figure 1: An overview of natural killer cell development.
NK cells derived from the CLP differentiate into a pre-NKP population, identified by its expression of CD117 and lack of CD122 expression. After becoming an NKP, the cells start expressing NK cell markers (NK1.1 and NKp46) and are considered to be immature NK cells at this stage. As they mature further, they acquire CD49b and CD11b expression and lose the expression of CD27. Fully mature NK cells are able to express cytolytic molecules and cytokines (including IFN-γ). Transcription factors also play important roles in governing lymphocyte fate from the CLP. A simplified list of transcription factors driving the NK cell lineage is shown in the box and the numbers on the diagram indicate where they have been identified at the different stages during development. ‘Early’ and ‘Late’ indicates when they are thought to be important during the maturation process. CLP; common lymphoid progenitor; HSC: haematopoietic stem cell; NK: natural killer; NKP: NK cell precursor.
Along with the expression of various cell surface markers and receptors, there are complex networks of transcription factors that can help dictate lymphocyte lineage commitments and give rise to distinct cell fates. Thymocytes can be diverted into an NK cell-like lineage if Bc111b, a Notch-1-dependent transcription factor, is ablated during T cell development.19 Furthermore, NK cells and other helper ILC, but not T or B cells, require Id2 and Nfil3 for their development.16
NATURAL KILLER CELL FUNCTION
The effector function of NK cells is determined by an integration of numerous signals. To sense their environment, NK cells use a tightly regulated balance of activating and inhibitory germline-encoded receptors, and initiation of an NK cell response is dependent on signalling via these receptors (Figure 2). Under physiological conditions, circulating NK cells are mostly in a resting state; however, activation by an array of cytokines can lead to the infiltration of these cells into pathogen-infected or cancerous tissues.
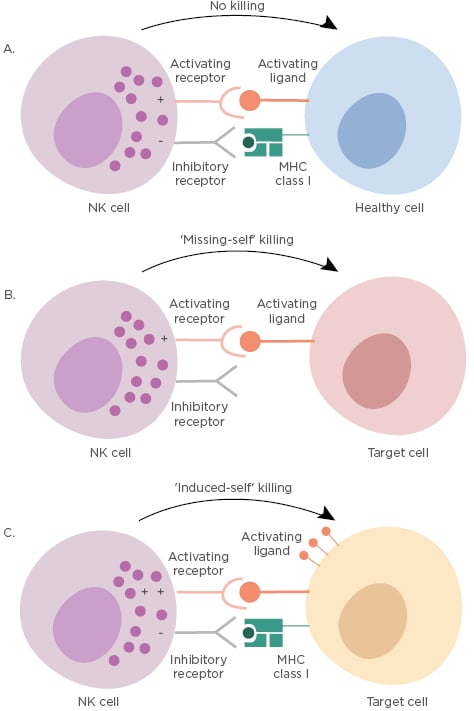
Figure 2: Schematic of natural killer cell function during ‘resting’ and ‘killing’ states.
K cells are able to recognise and kill target cells by an integrated balance of activating and inhibitory signals, which allow them to distinguish between healthy cells and target cells (those virally infected or transformed). A) ‘No-killing’ NK cells have balanced activating and inhibitory signals when recognising healthy cells. The inhibitory signals are delivered by self-MHC class I in this setting. B) Target cells, such as those infected or transformed, often downregulate or lose MHC class I molecules on their surface and these cells are detected by the NK cells as ‘missing-self’ leading to killing of the target cell. C) Tumour cells and virally infected cells often overexpress activating ligands on their surface, which are recognised by activating NK cell receptors; this process overrides any inhibitory signals triggering ‘induced-self killing’ and lysis of the target cell. A ‘+’ symbol indicates activating signal and a ‘-’ symbol indicates inhibitory signal. MHC: major histocompatibility complex; NK: natural killer.
Healthy cells express major histocompatibility complex class I (MHC I) molecules that act as ligands for inhibitory receptors on NK cells and contribute to the ‘self-tolerance’ of these cells. Killer cell immunoglobulin-like receptors in humans or members of the Ly49 family in mice make up the main inhibitory receptor profile of NK cells that bind MHC I molecules and maintain a tolerance for healthy host cells.20 However, during viral infection and tumour development, MHC I molecules are usually downregulated, lowering the inhibitory signalling threshold of the NK cell and leading to cell activation.21 Cellular stresses associated with infection or cancer growth, such as DNA damage responses and the expression of tumour suppressor genes, cause the upregulation of activating receptors on NK cells.
In most cases, NK cells are governed by a number of receptors and regulated by the integration of co-activating (NKp46, NKG2D, CD16, and LFA-1) and co-inhibitory (NKG2A, KLRG1, and TIGIT) signals via surface receptors that recognise the appropriate ligand.22 Signalling via the activating receptors on NK cells causes a shift in the balance towards activated NK cells that are able to directly eliminate target cells through NK cell-mediated cytotoxicity, or indirectly via the secretion of pro-inflammatory cytokines. There are also cytokine receptors that transmit activating (including IL-2, IL-12, IL-18, Type 1 IFN, and TNF-) or inhibitory signals (TGF-) during NK cell activity.22 Additionally, ligand interactions with cell–surface receptors on NK cells can lead to the secretion of pro-inflammatory cytokines, including IFN-.23
NATURAL KILLER CELL MEMORY
The quick response capabilities and enhanced host protection against a previously encountered pathogen make up the classical definition of immunological memory.24 Much like T cells, NK cells are able to acquire functional qualities associated with immunological memory in both non-infection settings and in response to pathogens.
During different educational routes, the formation of NK cell memory can occur in two ways: via antigen-dependent (virus or hapten-induced) or antigen-independent (cytokine-induced) mechanisms. Sensitising mice with haptens (molecules able to stimulate the production of antibodies) in the presence of the pro-inflammatory cytokines IL-12, IFN-, and IFN- leads to hapten-specific memory NK cells.25,26 Long-lived memory cells are generated following an infection and show a heightened response upon secondary challenge with the same pathogen. The memory formation process in T cells has been well characterised and is usually distributed into three main phases.27 Upon cognate antigen exposure, naïve T cells clonally expand and differentiate into effector T cells during the ‘expansion’ phase. This is followed by a second ‘contraction’ phase, in which most of the effector cells undergo apoptosis, leaving a small pool of stable T cells that can enter the ‘memory’ phase. These memory T cells persist throughout the organs of the host and maintain their longevity through self-renewal until they encounter their cognate ligand, where they display enhanced host protection and effector function.28 As cells of the innate immune system are unable to undergo somatic rearrangement of their receptor genes, it was thought that these cells, including NK cells, lacked antigen specificity and were, therefore, unable to develop classical immunological memory.14
However, in the common inbred laboratory mouse (C57BL/6), the activating receptor, Ly49H, which is expressed on approximately 50% of NK cells, binds with precise specificity to the mouse cytomegalovirus (MCMV)-encoded glycoprotein m157 expressed on infected cells to drive the expansion of virus-specific NK cells during the acute phase of MCMV infection.29,30 Once the infection is under control, expanded effector NK cells undergo a contraction phase to form a pool of long-lived, self-renewing ‘memory’ or ‘adaptive’ antigen-specific NK cells, a response similar to that observed in T cells. These NK cells can be recovered months after the initial infection in a number of peripheral tissues.31 The expansion and memory formation of virus-specific NK cells is dependent on an interaction with the viral antigen, as MCMV lacking the m157 glycoprotein does not induce Ly49H NK cell expansion or the development of memory.31 Previous studies have also shown that memory NK cells have a unique transcriptional signature when compared to naïve NK cells24 and possess functional attributes commonly associated with memory T cells, including secondary expansion, enhanced effector function, and increased protection against viral challenge.31 However, until recently, little was known about how transcription is controlled at an epigenetic level in NK cells while they transition between naïve, effector, and memory states. Chromatin accessibility analysis by assay for transposase-accessible chromatin using high-throughput sequencing (ATAC-seq) and transcriptional profiling via RNA-seq showed that, during MCMV exposure, NK cells undergo dynamic changes in chromatin architecture and that NK cells and CD8+ T cells share common epigenetic and transcriptional programmes as they transition from naïve to memory cells.32
NATURAL KILLER CELLS IN VIRAL INFECTION
NK cells play an important role in viral clearance but their responses were initially thought to be non-specific and lacking an immune memory response.33 However, it is now understood that NK cells are able to respond specifically to an infection and, in many cases, are able to develop memory recall responses.33 Inflammatory states can cause NK cells to enter the lymph nodes and influence T cell responses by promoting Th1 cell polarisation through the release of IFN- or restricting the expansion of T cells by killing activated cells.34 In healthy individuals, NK cells contribute to controlling several viral infections, including cytomegalovirus (CMV), influenza, hepatitis C, and HIV-1.35 Humans with complete or partial impairment in NK cell numbers or function have also been shown to have increased susceptibility to viral infections, including herpes simplex virus, CMV, varicella zoster virus, and human papillomavirus.35
During viral infection, NK cells use a number of approaches to sense inflammatory signals and express receptors for cytokines, including IFN-, IL-12, IL-15, and IL-18, whose expression is greatly upregulated during early infection, providing a vital role in the activation of NK cells and host protection.36 Many cytokine receptors are uniformly expressed by NK cells, suggesting cytokines are able to signal to most NK cells and in some cases activate the entire NK cell compartment.4 IL-12, IL-15, and IL-18 also provide important stimuli for the expression of IFN-, a hallmark of NK cell expansion in humans seropositive for human CMV (HCMV).37 Type I interferons, IFN- and IFN-, have also been shown to play an important role in increasing the cytotoxicity of NK cells38 while protecting NK cells from fratricidal killing, leading to enhanced defence and cellular expansion.39 Furthermore, mouse IL-12 induces epigenetic remodelling in regulatory regions of genes encoding transcription factors such as Zbtb32, Runx1, and Runx3, thereby contributing to NK cell expansion during MCMV.40 Along with the expression of cytokine receptors, NK cells express low-affinity Ig-G receptor FcRIII (CD16), allowing NK cells to bind to and become activated by antibody-coated target cells, a process known as antibody-dependent cellular cytotoxicity.41 This antibody-dependent activation of NK cells has been identified in response to a number of viruses, including influenza, HIV-1, and CMV.42-44
As discussed previously, NK cells possess a large number of activating and inhibitory receptors that are known to play important roles in controlling viral infection. The majority of NK cells express the activating receptors NKG2D, DNAM-1, NKp46, and, in humans, NKp30. The ligand for NKG2D is upregulated following environmental cues, such as cellular stress caused by viral infection, and this ‘induced-self’ recognition allows for NK cells to broadly survey for stressed cells and remove unhealthy or harmful host cells.45 NKG2D also provides a co-stimulatory signal to enhance proliferation and effector responses of NK cells during MCMV infection; however, NKG2D alone is unable to drive a robust expansion.46 Furthermore, ligands for DNAM-1 are upregulated during cellular stress but, again, this signal alone is not sufficient to expand NK cell subsets.47 Thus, co-stimulatory functions are needed for optimal expansion and differentiation of Ly49H+ NK cells during MCMV. Along with stress-induced receptor signals, other activating receptors are expressed to precisely sense viral signals including NK1.1, which is found on the majority of NK cells and recognises the MCMV encoded protein m12,48 or NKG2C (human) and Ly49 family (mouse) receptors on specific subpopulations of NK cells that are activated by an interaction with their cognate viral ligands.11 During HCMV infection, viral peptides derived from UL40 and presented on HLA class I histocompatibility antigen, alpha chain E and recognised by NKG2C, inducing population expansion of NKG2C+ NK cells.49 During MCMV infection in mice, the viral protein m157 is expressed on the surface of infected cells and recognised by the Ly49H receptor.50
NATURAL KILLER CELLS IN CANCER
NK cells were first identified for their ability to kill tumour cells without prior sensitisation. They are able to directly kill tumour cells through the release of cytotoxic granules containing granzyme and perforin.51 NK cells and cytotoxic CD8+ T cells work together to generate an immune response against viruses and tumour cells; however, tumours often downregulate MHC I, making them unrecognisable to cytotoxic T cells, leading to a failure to initiate adaptive immune response functions.52 The lack of MHC I expression or an upregulation of NKG2D ligands or CD70 (the ligand for CD27) can still render tumour cells susceptible to NK cell-mediated lysis.53 NK cells play vital roles in eradicating tumour cells and numerous studies have shown this in vivo by implanting tumour cells into mice genetically lacking NK cell function or by the administration of antibodies to deplete NK cells.54 In most cases, eliminating NK cells in these mice led to more aggressive tumour growth and metastasis.54 NK cells are also able to exert cytotoxicity against an array of malignancies, including acute myeloid leukaemia, acute lymphocytic leukaemia, and multiple myeloma, along with many solid tumours, including ovarian and colon tumours, and neuroblastomas.49,55
NK cells can be activated by various stimuli, including contact with DC. DC are the main antigen-presenting cells of the immune system and play a fundamental role in sensing pathogens and initiating an immune response. A bi-directional crosstalk between DC and NK cells has been observed in secondary lymphoid tissues and in the periphery via cell–cell contacts or the release of soluble factors.56 These interactions can result in cellular activation and maturation, and the production of cytokines by both cell types.57 Additionally, NK cells are able to directly or indirectly regulate T cell responses because NK cell-mediated killing can cause the release of antigens, which are able to enhance CD8+ T cell activation by cross-presentation on DC.58 The tumour microenvironment contains transformed cancer cells along with stromal cells that are able to control tumour progression. Recent findings have shown that human NK cells directly identify the ligand platelet-derived growth factor DD (PDGF-DD) by using the activating receptor NKp44, which, in turn, enables NK cells to limit tumour growth.59 This PDGF-DD–NKp44 interaction stimulates NK cell secretion of IFN-, TNF-, and chemokines. The cytokine production triggers tumour cell–cycle arrest.59 Conversely, cancer cells are able to evade the immune response and NK cells by using a number of mechanisms; these include increasing MHC I molecules to inhibit NK cell function, decreasing NKG2D ligand expression to impair NK cell recognition, and upregulating levels of inhibitory cytokines (such as IL-10 or TGF-) in tumours following secretion by the tumour itself, regulatory T cells, or myeloid derived suppressor cells.60
Although it has been known for some time that NK cells play a key role in fighting tumour development and progression, in more recent years NK cell-based immunotherapy has become a novel and promising approach to treating tumours.61 Preclinical and clinical studies have focussed on host NK cells and their antitumour function, and administration of activating cytokines (IL-2 and IL-15) have shown mixed results.62 IL-2 treatment in mice was shown to be efficacious in improving antitumour responses and was approved for clinical use in a number of human cancer types;63 however, following injections of IL-2, tumour relapse and survival rates were unaltered in some settings.64 IL-15 treatment became an alternative to IL-2, and using IL-15/IL-15R complexes to reflect trans-presentation of IL-15 physiologically,65 and, in combination with other immunotherapies, have also become a main focus of many studies.
CONCLUSION
NK cells are a key component of the immune response and play vital roles in controlling and eliminating both virally-infected and cancer cells. Although our knowledge of basic NK cell biology and innate immunity continues to grow rapidly and many studies have shown that the development and function of NK cells is highly dynamic, there is still much to be investigated. The effector function of these NK cells must be further studied, with a predominant focus on immunotherapies along with the prevention of infectious diseases and cancer. Furthermore, the discovery of NK cell immunological memory and epigenetic reprogramming during infection has led to many thought-provoking and exciting questions regarding both innate and adaptive immune responses.





